Aicardi-Goutières syndrome (AGS)
Aicardi-Goutières syndrome (AGS) is a genetic condition that resembles a congenital infection such as HIV infection or one of the TORCH infections (see below). This resemblence appears to be due to high levels of a substance called type 1 interferon in both AGS and congenital infections (1). This substance is produced by the body as a tool to fight viral infections. While it is a critically important part of the immune system, too much of it (especially at the wrong time in a person's development) can cause serious health problems. Most people with AGS have high or very high levels of type 1 interferon in their spinal fluid and/or blood when the syndrome starts to show itself. For this reason, AGS is called a type 1 interferonopathy, or a condition where abnormally high levels of type 1 interferon are central to the development of disease (1, 2).
Other members of the interferonopathy group include systemic lupus erythematosus (SLE), spondyloenchondrodysplasia (SPENCD), and Singleton-Merten syndrome, among others (see references 2 and 3 for a list). Cree encephalitis, a condition that affects the Cree peoples of Canada and Montana, is now recognized as being a form of AGS (4).
Clinical information
The onset of AGS tends to fall into three broad categories: prenatal (signs are evident before or at birth), postnatal (infant is normal at birth; signs appear in the year of life, often at ages 3-7 months), and later onset (signs appear later, though usually in childhood; 5, 6). One of the chief signs of AGS is encephalopathy, or overall brain illness. The exact problems that occur with encephalopathy vary, but after birth in AGS, they include irritability (may be severe), skill loss, slowing of head growth, and occasional fevers in the absence of infection (7). Overall, however, the course of disease in most patients is marked by an initial period of regression (loss of skills) followed by stabilization (7, 8).
We have listed common clinical features of AGS below. For a description of findings that are suggestive of AGS, see the Diagnosis section, below.
- Delayed development of any gross motor skills (head control, sitting, walking, etc.)
- Tight muscles in the extremities and loose/floppy muscles in the trunk
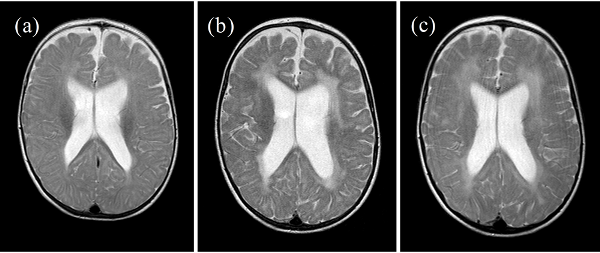
- Changes to brain white matter on MRI ("leukodystrophic changes")
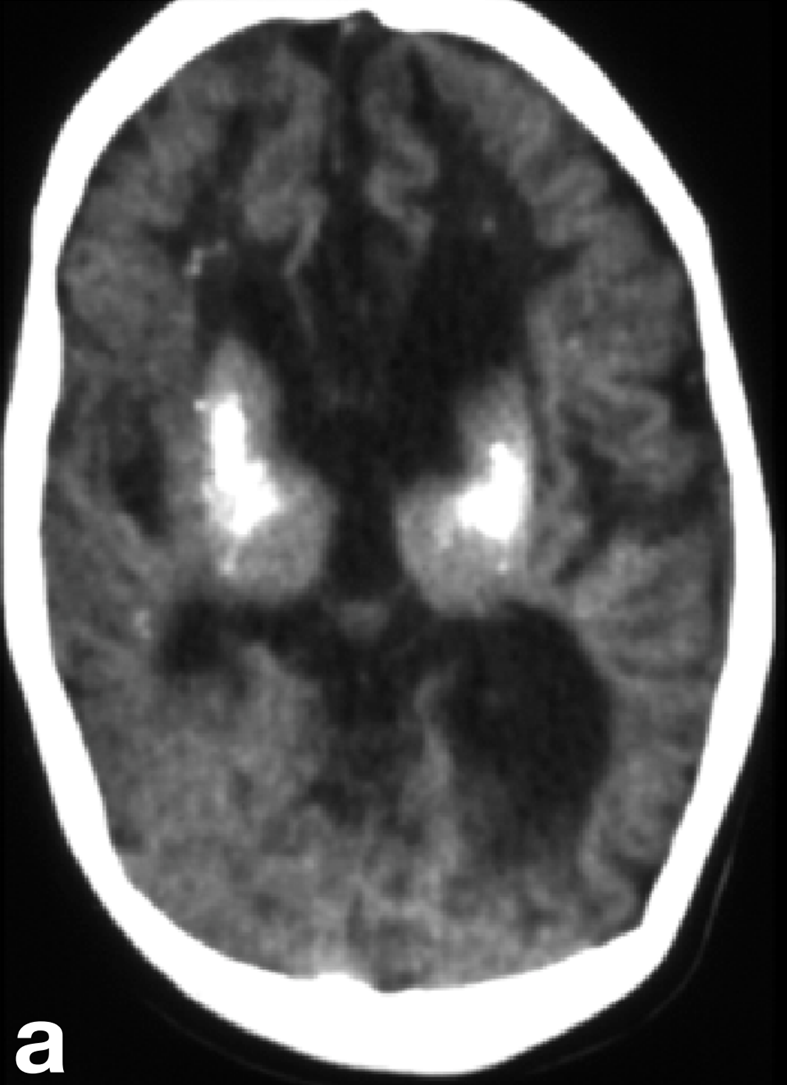
- Brain calcifications (calcium deposits in a patient's brain tissue)
- Encephalopathy (brain disease) in an infant/very young child
- Dystonia (muscle spasms that cause abnormal posturing)
- Failure to thrive (poor weight gain in an infant/child)
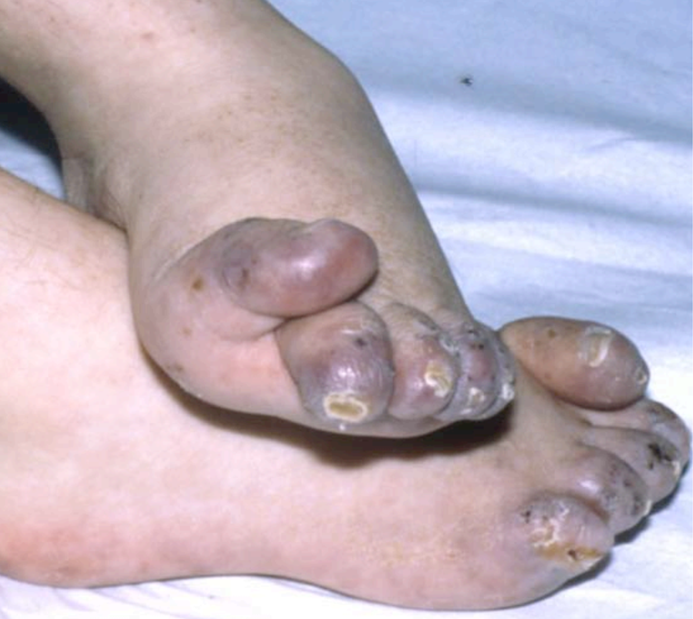
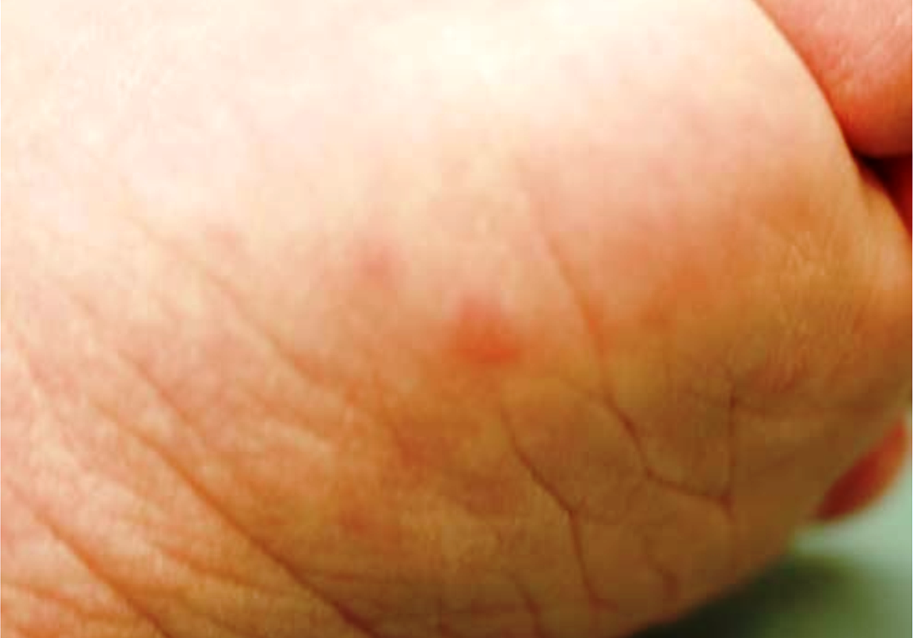
- Chilblains or chilblain-like skin lesions (see photos)
- Impairment of vision (glaucoma, cortical blindness)
- Neurological problems that get worse with time
- Irritability, especially in an infant/toddler
- Loss of milestones (e.g. motor skills)
- Intellectual disability/very low IQ
- Microcephaly (very small head)
- Delayed speech development
- Feeding problems
- Short stature
- Abnormally high levels of interferon alpha (CSF, possibly blood)
- Abnormally high numbers of white blood cells (CSF)
- Elevated levels of immunoglobulins G & M (blood)
- Abnormally high levels of neopterin (CSF)
Common clinical features of AGS
Signs and symptoms
Abnormal laboratory values
Cause
AGS is associated with several genes: TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR/DRADA, and IFIH1/MDA5. It is usually an autosomal recessive disorder. This term means that a disease is caused by a mutation in a gene not located on an X or Y chromosome, and that a baby will have only have it if each parent contributes a copy of the mutated gene. However, some forms of AGS are autosomal dominant, meaning that a mutation in one copy of a gene can cause disease. In the case of dominant mutations in TREX1, mutations often arise sporadically and a parent is not affected.
Diagnosis
The key features for diagnosing AGS include encephalopathy and/or intellectual disability, microcephaly in the first year of life (usually not present at birth), fevers in the absence of infection, enlargement of the liver and spleen, mottled skin and/or chilblain lesions (see photos; may occur on hands, feet, ears), and muscle spasticity and dystonia (7). In addition, suggestive imaging findings include brain calcifications and white matter abnormalities (7). Importantly, calcifications may not be visible on MRI, which is often the first imaging test used in a patient presenting with AGS-like problems. In addition, chilblains are not always present --- for example, in our literature survey, 35% of 130 patients had chilblains or chilblain-like lesions.
Laboratory testing helps support a diagnosis of AGS. For example, in the early stages of disease, levels of interferon-alpha (IFN-a) are high in the CSF. (normal: less than 2 IU/ml). Importantly, however, IFN-a levels can normalize by ages 3 to 4 and may be normal in the blood. In addition, patients have chronically elevated levels of lymphocytes in their CSF, which, like IFN-a, may normalize with time. Finally, AGS patients may have elevated levels of neopterin in the CSF. Again, levels may normalize with time. Reference 7 notes that levels of neurotransmitter metabolites 5HIAA, HVA, and 5MTHF are normal.
Identification of a mutation in one of the genes associated with AGS can provide a definitive diagnosis. For more information about diagnosing AGS, see reference 7.
Differential Diagnosis
TORCH congenital infections. TORCH stands for Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV),and Herpes infections.TORCH infections cause medical problems that are similar to those of AGS. In addition, congenital infection with HIV-1 and Zika virus can cause clinical features similar to those seen in AGS. These infections must be ruled out in a patient with AGS-like clinical features.
Leukoencephalopathies. The leukoencephalopathies are diseases that feature abnormalities in the brain's white matter. Unfortunately for diagnosis, these abnormalities tend to result in an overlapping set of problems, such as delayed development of motor and speech skills, intellectual disability, seizures, ataxia, and other problems. As a result, the clinical features overlap in a number of diseases, a fact that makes it difficult to narrow the field and obtain a diagnosis. Fortunately, there are strategies that can help. For example, one recent publication outlined patterns in white matter abnormalities among different diseases (9). The information in that paper may help clinicians trying to diagnose a patient with white matter abnormalities.
Cockayne syndrome (CS). CS is a leukoencephalopathy that causes a variety of problems also seen in AGS. They include brain calcifications, white matter abnormalities, intellectual disability, microcephaly, feeding problems, failure to thrive, and muscle spasticity. However, CS patients are photosenstive and most burn easily. Other problems that occur in CS patients but not in AGS include cataracts and pigmentary retinopathy.
Band-like calcification polymicrogyria. (BLC-PMG or Pseudo-TORCH syndrdome). Patients with this very rare syndrome have brain calcifications, developmental delays, and seizures. It differs from AGS by the presence of polymicrogyria, which is an abnormality of brain anatomy in which the brain has an excess of very small folds. BLC-PMG is caused by mutations in the gene OCLN.
Other conditions in the differential diagnosis for AGS are listed in reference 7, which is freely available on the internet.
References
- 1. Crow Y & Manel N (2015) Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol 15(7):429-440. Abstract on PubMed.
- 2. Crow Y (2011) Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci 1238:91-98. Abstract on PubMed.
- 3. Volpi S et al. (2016) Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J 14(1):35. doi: 10.1186/s12969-016-0094-4. Full text on PubMed.
- 4. Crow YJ et al. (2003) Cree encephalitis is allelic with Aicardi-Goutières syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet 40(3):183-187. Full text on PubMed.
- 5. Crow YJ et al. (2015) Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 167A(2):296-312. Full text on PubMed.
- 6. Chahwan C et al. (2012) Aicardi-Goutieres syndrome: from patients to genes and beyond. Clin Genet 81(5):413-420. Abstract on PubMed.
- 7. Crow Y (2005) Aicardi-Goutières syndrome. Updated November 22, 2016. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 8. Rice G et al. (2007) Clinical and molecular phenotype of Aicardi-Goutières syndrome. Am J Hum Genet 81(4):713-725. Full text on PubMed.
- 9. Schiffmann R et al. (2009) Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 72(8):750-759. Full text on PubMed.
- 10. Crow YJ (2006) Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat Genet 38(81):910-916. Abstract on PubMed.
- 11. Crow YJ (2006) Mutations in the gene encoding the 3'-5' DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat Genet 38(81):917-920. Abstract on PubMed.
- 12. Annastas A (2016) Photo: Wyatt Gillette's Honorary Marine Ceremony Official US Marine photo on Wikimedia Commons. The appearance of U.S. Department of Defense (DoD) visual information does not imply or constitute DoD endorsement.
- 13. Tungler V et al. (2014) Phenotypic variability in a family with Aicardi-Goutières syndrome due to the common A177T RNASEH2B mutation. Case Rep Clin Med 3(3):43716. doi:10.4236/crcm.2014.33037. Article on publisher website.