Creutzfeldt-Jakob disease (CJD) and other prion diseases
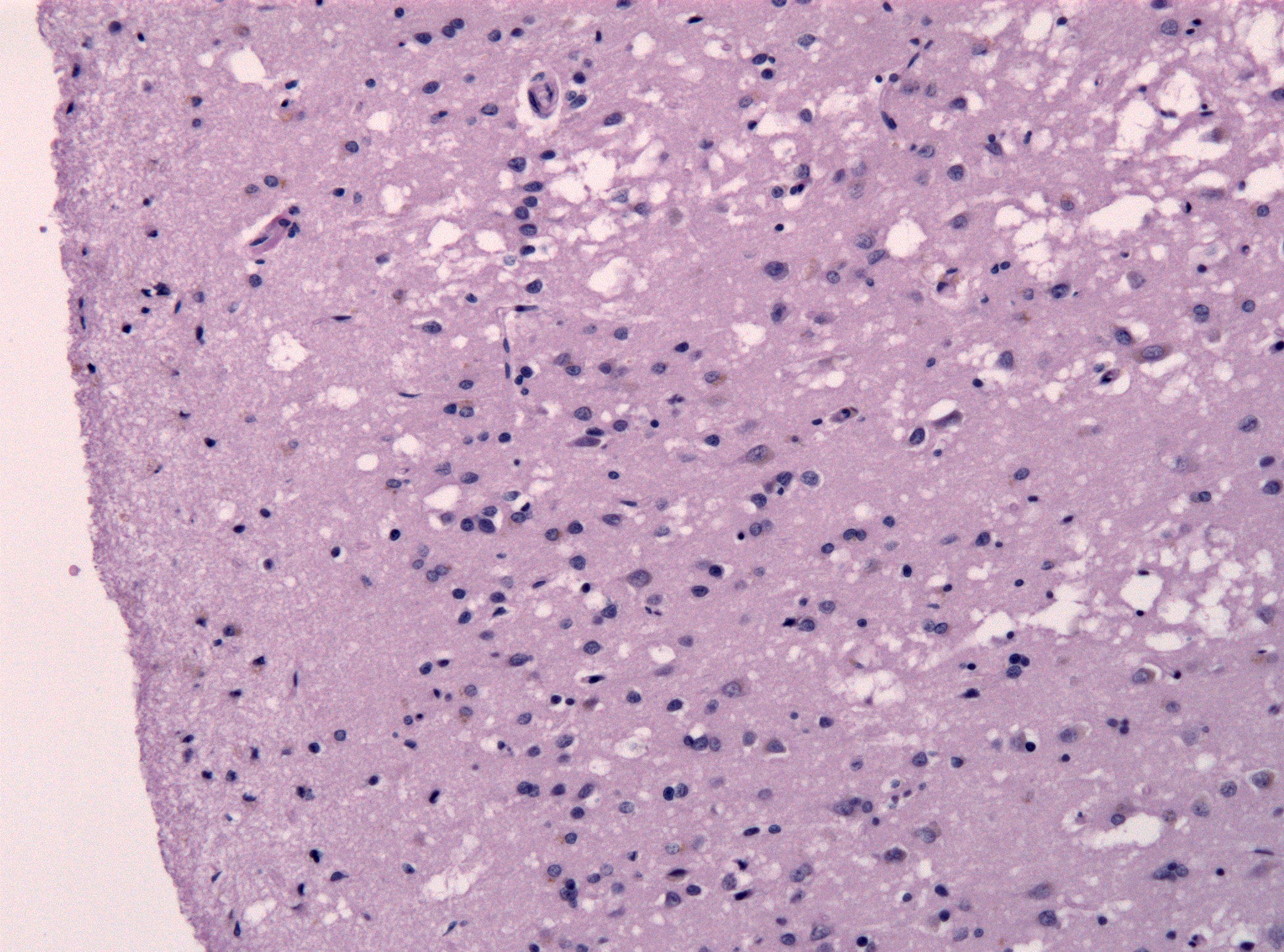
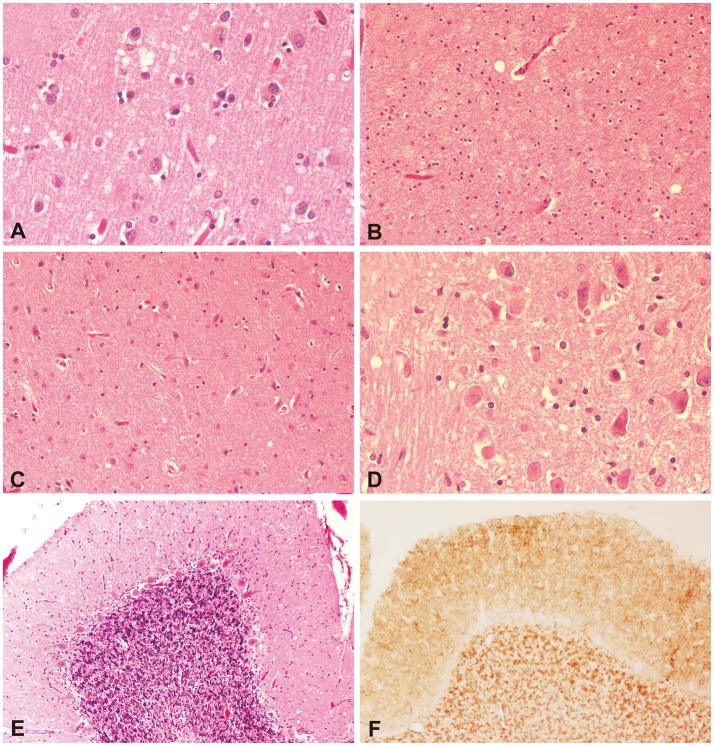
Creutzfeldt-Jakob disease (CJD) is the most common member of a group of diseases called human transmissible spongiform encephalopathies (TSEs). Spongiform means that when viewed under a microcsope, a brain specimen from a CJD patient looks like a sponge (see photo below on right). Encephalopathy is a broadly defined term that means abnormal brain structure or function. Transmissable means that these diseases are contagious. However, they cannot be passed from one person to another through the air, by touching, or through other forms of casual contact. See the section on Transmission below for more information. TSEs are progressive disorders, which means that they get worse with time.
Other members of the TSE group include Gertstmann-Sträussler-Scheinker disease (humans), kuru (humans), fatal familial insomnia (humans), scrapie (sheep, goats and mouflon), transmissible encephalopathy (mink), chronic wasting disease (deer and elk), and bovine spongiform encephalopathy (cows; BSE).
As a group, prion disorders are extremely rare, with a major study of 11 countries around the world finding 1.67 annual deaths per million people (1). The same study found that deaths from CJD were 1.39 per million, which highlights the fact that CJD is the most common member of the group.
Clinical information
CJD has a long incubation period that may last for decades. However, once medical problems begin to occur, they get worse quickly or very quickly. CJD is always fatal. The Merck Manual states that most people die with six months of diagnosis, although a minority may live for 2 years or more. Most people with CJD develop problems with cognitive skills in the early stages of disease (memory, attention, speech, etc.), although psychological problems also occur. The problems listed below are early signs of the most common forms of CJD (sporadic and familial; discussed below). These problems do not necessarily occur in each patient as an early symptom.
- Disorientation or confusion about time
- Disorientation or confusion about place
- Memory loss or forgetfulness
- Hyperintensities on MRI images
- Loss of cognitive abilities
- Abnormal EEG (see figures)
- Personality changes
- Behavior changes
- Hallucinations
- Unsteady gait
- Depression
- Problems get worse
Common early problems in sporadic or familial CJD
Other signs and symptoms include weakness on one side of the body, hallucinations, myoclonus (sudden involuntary jerking of a limb), vertigo, and hallucinations.
People with CJD may be misdiagnosed initially as having depression. A key distinction between depression and CJD is that that CJD patients have significant neurological problems that get worse with time. They also have problems that are not typically associated with depression, such as impairments in muscular coordination including gait abnormalities, and speech difficulties, including dysarthria and aphasia/dysphasia.
As CJD progresses, patients lose both motor skills and cognitive abilities. Problems that tend to occur later in the course of the disease are as follows:
- Coma
- Dementia
- Loss of ability to walk
- Akinetic mutism (patient does not move or speak)
Common problems that occur late in the course of CJD
CJD is associated with mutated proteins called prion proteins. Prion proteins are abundant in the nervous sytem. In their normal state, they appear to have a role in maintaining myelin sheaths (2), making them important for maintaining health. Myelin is a fatty substance that surrounds parts of nerve cells. It acts as an insulator and it helps send signals from the nervous system to the rest of the body. Problems develop when prion proteins become abnormally shaped because of mutations in the gene PRNP. The precise mechanisms by which abnormal prions cause disease are not known. However, it is known that they cannot be broken down in the way that normal prion proteins are, and that they accumulate in the brain as a result. Researchers believe that this process ultimately destroys neurons and leads to the problems described above. For reasons that are not understood, it can take decades for the symptoms of CJD to appear.
There are a number of forms of CJD. The most common one is the sporadic type. Sporadic means that CJD cases occur with no known cause. This form represents roughly 85-90% of all cases of CJD. Sporadic CJD tends to occur more often in people in late middle age. Although rare, there are cases with onset below age 50, however.
The second largest group of CJD cases are genetic, or familial CJD. This group accounts for 10-15% of all cases. These cases are caused by mutations in a gene called PRNP. Familial CJD is an autosomal dominant disease, meaning that it is passed from one affected parent to a child. The child of a person with familial CJD has a 50% chance of inheriting the disease. The medical problems associated with familial CJD are generally similar to those of sporadic CJD, although the familial cases may have an earlier onset.
There are two final types of CJD: iatrogenic (due to medical or surgical treatment) and variant (caused by eating meat products from infected animals). Both of these forms are caused by transmission of the abnormal protein from an infected person or animal to another person. Iatrogenic transmission does not appear to occur anymore, and methods to stop the spread of variant CJD are ongoing and apparently successful.
Iatrogenic CJD resulted from the use of contaminated growth hormone in children and from contaminated grafts of the dura mater in certain surgical patients. The dura mater is a leathery membrane surrounding the brain and spinal cord. These grafts are sometimes necessary after injuries or brain surgery. Dura mater grafts from deceased donors was used, and they infected recipients. In addition, a very small number of cases were caused by the use of contaminated corneas for transplantation and through the use of contaminated medical instruments (heating/cooking does not destroy abnormal prion proteins). Iatrogenic CJD is a very rare form of CJD, accounting for a total of 469 cases as of mid-2012 and a further case reported in 2013 (3,4). Thanks to awareness of the problem and new sterilization methods that destroy prion proteins on surgical instruments, it is now very unlikely that new cases will appear, with the exception of cases where infection occurred decades ago.
Variant CJD can be acquired by eating diseased brain, spinal cord, or other nervous system tissue from cows afflicted with BSE (mad cow disease). Slaughtering practices that mixed material from the nervous system resulted in the inclusion of infectious material in meat sold for human consumption. As noted above, heating/cooking does not destroy abnormal prion proteins. The cows had become infected because they had been given protein supplements and/or feed made from by-products that came from sheep with scrapie or other cows with BSE. These feeding practices have now been discontinued. Many cows were slaughtered while showing obvious signs of disease, a practice that is now frowned upon, but may continue.
Variant CJD first came to light in the mid 1990s in the United Kingdom when clusters of the disease began to appear in young people, some of whom lived in the same small villages. Given that CJD generally kills one person per million, the clusters were a sign that something was very wrong. After investigations and advocacy by families of patients, the condition was definitively linked to the consumption of meat from cows with BSE. To date, over 183,000 cases of BSE have been diagnosed in cows in the United Kingdom (source: Wikipedia). Out of a total of 229 cases of variant CJD, 177 have occurred in the UK, with only 1 death in the years 2012-2015 (5). The second highest number of cases has been in France, with 27, followed by Spain, with 5.
The early clinical features of variant CJD are somewhat different from those of sporadic and familial CJD. Most importantly, patients tend to be younger. In contrast to sporadic CJD, where onset below age 50 is rare, the median age of the British patients was 28. It must be noted that age is not a guarantee against variant CJD, however, as the oldest patient was 74 years old.
In the first 100 vCJD patients, psychiatric problems tended to precede neurological signs (63% of cases; 6). Psychiatric problems in this group included depression, anxiety, agitation, and hallucinations/delusions. Any of these problems alone or in combination can lead to misdiagnosis of vCJD as a psychiatric disorder. Unfortunately, the generally rapid course of the disease soon highlighted the error.
It should also be noted that 15% of these 100 vCJD patients displayed neurological signs, while 22% of patients presented iwth both fact psychiatric and neurological problems. These facts highlight the variable nature of the disease. It is hoped that new animal feeding practices and efforts to eradicate BSE have stopped the appearance of new cases of vCJD.
Interested readers may wish to consult a detailed descriptions of the features of CJD (7).
Diagnosis and Testing
Definitive testing for familial CJD and other familial prion diseases can be performed with gene sequencing. Definitive diagnosis of the sporadic prion diseases requires a brain biopsy or brain autopsy speciman. Given that this type of test is highly invasive and risky, diagnosis is usually clinical (based on signs and symptoms). If a biopsy is taken, the usual findings are spongiform degeneration (see photo at right, above). This abnormality is distributed through the cortex and deep nuclei of the brain.
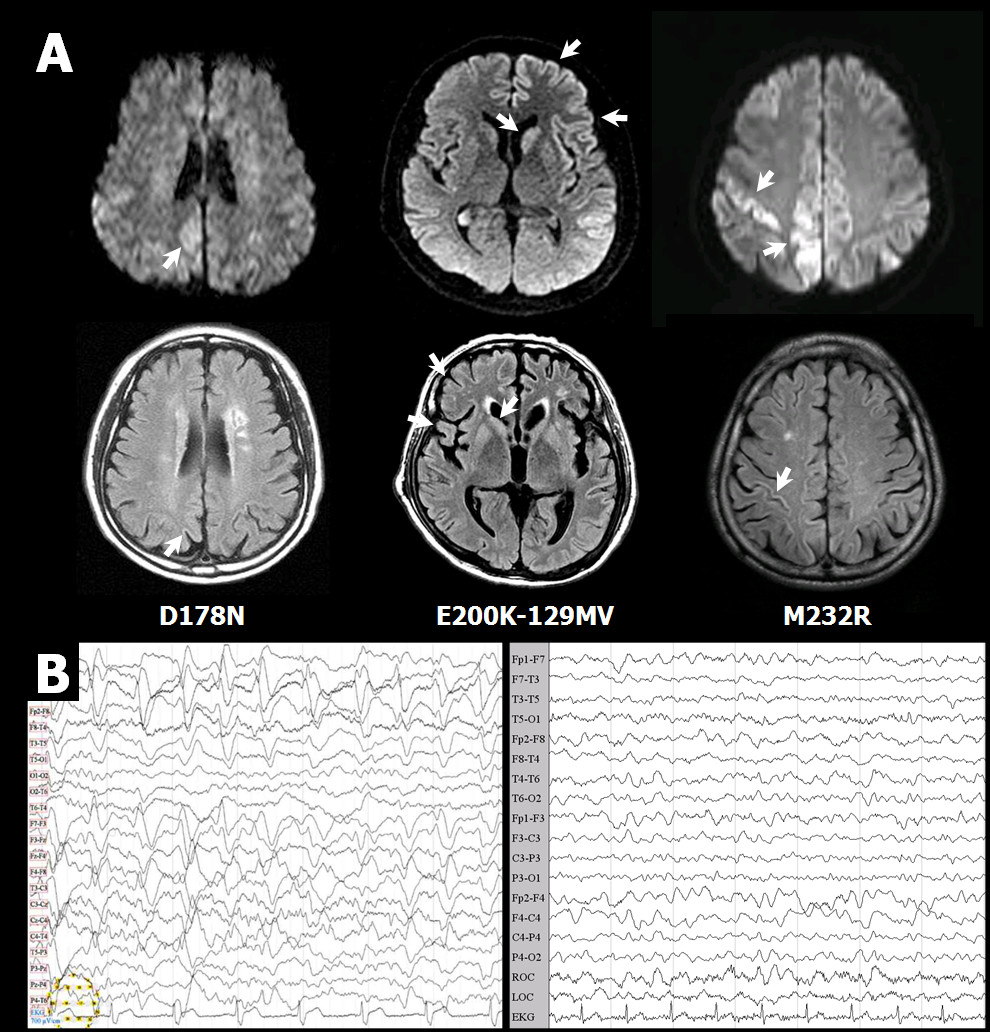
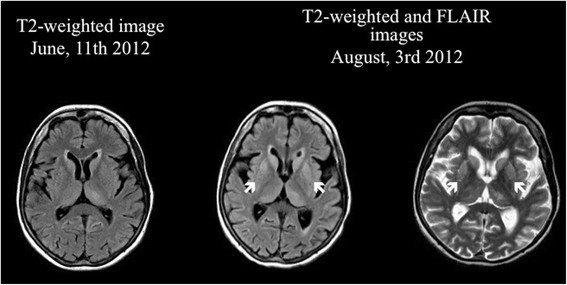
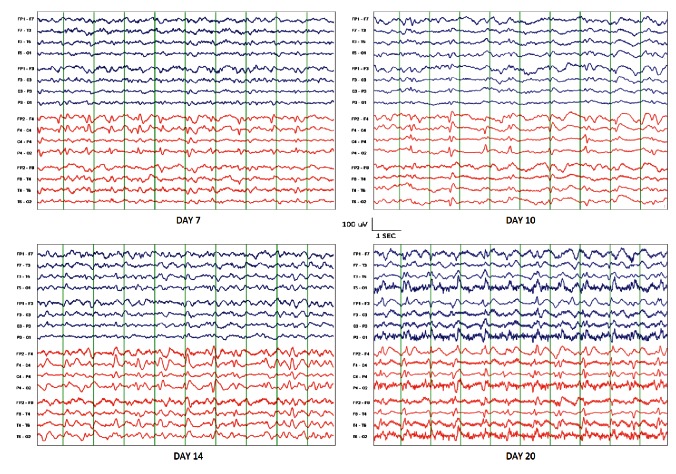
In addition to pathology tests, brain MRI imaging, examination of cerebrospinal fluid, and EEGs are also helpful (see the NIH GeneReviews article on prion diseases, linked to at right, for more information). The photos below show examples of abnormalities in brain images and EEGs of CJD patients.
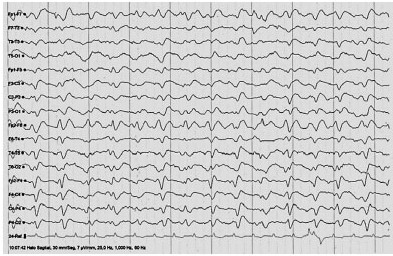
EEG readings and MRI images change as CJD progresses. In sporadic cases, early-stage EEGS tend to have non-specific findings, including diffuse slowing and rhythmic delta activity. In the middle and late stages, periodic sharp wave complexes are often observed (see photo below). These complexes are considered to be typical for the disease. Late stage patients may have areactive coma traces or even alpha coma in preterminal EEG recordings (8). This series of EEGs in a single patient over the course of many months highlights these changes. In addition, EEG changes can happen over the course of a matter of days in some patients (see figure below), and frequent readings may be necessary to aid diagnosis (9).
The US National Institutes of Health maintains a list of centers that can look for mutations in PRNP. (see link at right). If you are not a doctor and suspect that you or someone in your family has CJD or another prion disease, check with your family physician, who will compare signs and symptoms with those known to occur in CJD. Your doctor can obtain a sample from you and send it to a testing center.
Differential Diagnosis
Hashimoto's encephalopathy (HE). HE is an autoimmune condition whose pathogenesis is not well understood. It causes a condition that can resemble CJD very closely: patients may experience loss of cognitive abilities and dementia, personality changes, epileptic seizures, myoclonus, loss of motor skills, and alterations of consciousness. Like CJD, HE may be progressive. In some cases, however, and unlike CJD, it may relapse and remit. HE is more common than CJD.
One feature that is common to all HE patients described to date in the literature is the presence of high levels of antithyroid antibodies in the serum. They may also be present in the CSF, although this is not the case for all HE patients. An important fact about HE is that it typically responds to treatment with corticosteroids or other medications (see our HE page for more information).
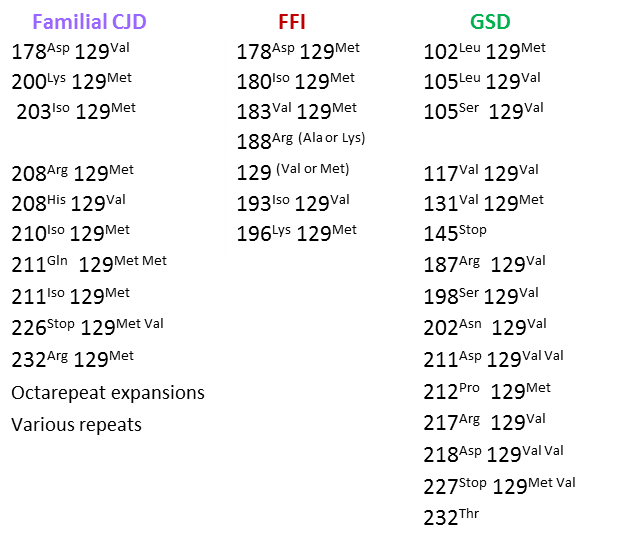
Other prion diseases. The differential diagnosis of CJD includes the other disorders in the human TSEs. One closely related TSE is fatal familial insomnia (FFI). FFI is a very rare condition that, like CJD, can be caused by mutations in the PRNP gene. Generally speaking, different mutations in the gene cause different diseases. However, the FFI or CJD can result from the same mutations in codon 178 of PRNP (the are called D178N or 178Asp). The difference in clinical outcomes is driven by a polymorphism in codon 129. Patients with a methionine amino acid residue at codon 129 and the 178Asp mutation have FFI, while patients with a valine residue at codon 129 and the 178Asp mutation have CJD (10).
Overall, FFI and CJD share many similarities. For example, as its name implies, FFI is strongly associated with insomnia. However, insomnia is not necessarily a presenting symptom. One study of 12 FFI and 11 CJD patients found that only 3 FFI patients had insomnia as a presenting symptom. Ten of the 12 FFI patients developed insomnia as an early problem, but 2 did not experience it at all (11). The study also stated that 2 FFI and 3 CJD patients presented with atxia, with 1 from each group presenting with memory loss. Other problems listed as presenting symptoms in both diseases were anxiety, depression, and visual disturbances. Another paper describing 5 FFI patients found that insomnia was documented in only 4 (12).
Early problems associated with FFI include psychological or cognitive changes, such as anxiety, depression, memory loss, or problems with specific cognitive tasks such as arithmetic or reading. Weight loss may also occur. In patients who develop insomnia, lack of sleep can result in phobias and paranoia that can progress to hallucinations and panic attacks. Like CJD patients, FFI patients also have memory loss, gait problems including ataxia, and suffer from confusion and a dementia-like state. Patients may become mute may transition to a hypersomnolent state in the late stage of the disease.
Importantly, in some cases described to date, insomnia and/or changes in sleep patterns did not become known until after a patient's death. This situation may be due to the insomnia being overlooked among a large set of overwhelming and progressive neurological problems. Patients with FFI may also have autonomic disturbances (rapid heartbeat, high blood pressure, hyperhidrosis/excessive sweating, and high body temperature).
The description above shows that there is considerable overlap between CJD and FFI. In fact, some researchers have suggested that they may be two extremes of a single disease spectrum rather than distinct, discrete conditions (11). Given that both FFI and CJD are fatal, and that there are no treatments available this time, the distinction may have little practical value in terms of patient needs (though it may be useful for purposes of understanding CJD and FFI).
Fatal insomnia can also be sporadic, but the vast majority cases described to date have been familial.
Gerstmann-Sträussler-Scheinker disease (GSS) is another TSE. Like FFI and CJD, it is also caused by mutations in PRPN. A sporadic form has not been described. As might be expected, GSS is clinically similar to CJD and FFI. Although it is also fatal, it generally progresses more slowly, with a course that averages 5-6 years (range: 3 months to 13 years; 13). GSS appears to be caused by mutations that are distinct from those associated with CJD and FFI (14). GSS is extremely rare, with an estimated prevalence of 1-10 patients per hundred million people (15). Clinically, it is characterized by progressive ataxia, Parkinsonian symptoms, and dementia (16).
The table below lists different mutations in PRNP and disease outcomes. Different disease outcomes presumably result from differences in how the abnormal PRNP protein folds, with different shapes causing different problems. For summary information about each mutation, including brief case histories, see reference 17.
Other conditions in the differential diagnosis of CJD include dementias (Alzheimer's disease, dementia with Lewy bodies), frontotemporal dementias, ALS, Huntington's disease, and the inherited ataxias. Other diseases in the differential diagnosis of CJD are described in the GeneReviews document linked to at the right.
Clicking on the link at the right will provide a list of clinical trials for CJD.
People who share a home with a prion patient are not at increased risk of becoming sick. Rather, these conditions are spread by consuming infected materials such as brain material (as happened with Kuru and native peoples of Papua New Guinea, and with people who ate infected materials from cows with variant CJD; see above). Other routes of transmission include transplantation of infected brain or nervous system components. Variant CJD has been spread through blood transfusions from asymptomatic infected donors in a small number of cases. It is not known if other forms of prion diseases can be spread through blood, but there laws and guidelines generally prohibit donations from high-risk individuals.
References
- 1. Ladogana A et al. (2005). Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 64(9):1586-1591. Abstract on PubMed.
- 2. Bremer J et al. (2010). Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci 100(13):310-318. Abstract on PubMed. Full text on ResearchGate.
- 3. Brown P et al. (2012) Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Inf Dis 18(6):901-907. Full text on PubMed.
- 4. Appleby P et al. (2013) Iatrogenic Creutzfeldt-Jakob disease from commercial cadaveric growth hormone. Emerg Inf Dis 19(4):682-684. Full text on PubMed.
- 5. UK National CJD Surveillance Unit (2015) Creutzfeldt-Jakob disease in the UK. Full text.
- 6. Spencer MD et al. (2002) First hundred cases of variant Creutzfeldt-Jakob disease: retrospective case note review of early psychiatric and neurological features. BMJ 324:1479-1482. Full text on PubMed.
- 7. UK National CJD Surveillance Unit (2012) The clinical features of human prion diseases. Full text.
- 8. Wieser HG et al. (2006) EEG in Creutzfeld-Jakob Disease. Clin Neurophysiol 117(5):935-951. Abstract on PubMed.
- 9. World Health Organization (1998) Global Surveillance, Diagnosis and Therapy of Human Transmissible Spongiform Encephalopathies: Report of a WHO Consultation. Full text from the WHO.
- 10. Goldfarb LG et al. (1992) Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science 258(2083):806-808. Abstract on PubMed.
- 11. Zarranz JJ et al. (2005) Phenotypic variability in familial prion diseases due to the D178N mutation. J Neurol Neurosurg Psych 76:1491-1496. Full text on PubMed.
- 12. Almer G et al. (1999) Fatal familial insomnia: a new Austrian family. Brain 122:5-16. Full text from publisher.
- 13. Collins S et al. (2001) Gerstmann-Sträussler-Scheinker syndrome, fatal familial insomnia, and kuru: a review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci 8(5):387-397. Abstract on PubMed. Full text on ResearchGate.
- 14. McKusick VA (1986) OMIM entry on Gerstmann-Straussler-Scheinker disease. (2014 update) Full text.
- 15. Kong Q et al. (2004) Inherited prion diseases. In: Prion Biology and Diseases. Prusiner SB (ed). Cold Spring Harbor Laboratory Press, New York. Book on amazon.com.
- 16. Aralasmak A et al. (2006) A prion disease-possible Gerstmann-Straussler-Scheinker Disease. A case report. J Comput Assist Tomogr 30(1):135-139. Abstract on PubMed. Full text from authors.
- 17. Liberski PP & Surewicz WK (2013) Molecular Genetics of Gerstmann-Sträussler-Scheinker Disease and Creutzfeld-Jakob Disease. Genetics 2:117. doi:10.4172/2161-1041.1000117 Full text.
- 18. Choi BY et al. (2009) Mutations at codons 178, 200-129, and 232 contributed to the inherited prion diseases in Korean patients. BMC Inf Dis 9:132 doi:10.1186/1471-2334-9-132 Full text on PubMed.
- 19. Zuhorn F et al. (2014) Creutzfeldt-Jakob disease mimicking autoimmune encephalitis with CASPR2 antibodies. BMC Neurol 14(1):227 doi:10.1186/1471-2334-9-132 Full text on PubMed.
- 20. Torres-Ramírez L et al. (2013) Creutzfeldt-Jakob disease in Peru: report of eleven cases. Rev Peru Med Exp Salud Publica 13(2):364-369. Full text from Scielo.
- 21. Mader EC et al. (2013) Sporadic Creutzfeldt-Jakob disease with focal findings: caveats to current diagnostic criteria. Neurol Int 5(1):e1 doi: 10.4081/ni.2013.e1 Full text on PubMed.
- 22. Sierra-Hidalgo F et al. (2014) Ocular Dipping in Creutzfeldt-Jakob Disease. J Clin Neurol 10(2):162-165. Full text on PubMed.
- 23. Gaillard F (2003). Wikimedia Commons. Open-source photo.