Cutis Laxa, Types 2A and 2B
Cutis Laxa (CL) is a term that refers to a group of related disorders that feature loose or sagging skin. All forms of cutis laxa are very rare. The skin problems in cutis laxa are the result of problems with connective tissue, which is tissue that supports, connects, or separates other tissues and organs. Connective tissue is found everywhere in the human body. In cutis laxa patients, connective tissue defects result in stretchy, inelastic skin that may sag and/or be slow to return to its normal position after being stretched. This characteristic is in contrast to Ehlers-Danlos syndrome patients, who also have stretchy skin. In EDS patients, stretched skin generally returns to its normal position after being released.
In addition to causing skin problems, connective tissue defects also create internal problems in cutis laxa patients. Internal tissues are often weak and unable to support organs. This situation leads to hernias and other problems such as diverticula (a pouchlike protrusion from an organ such as the bladder).
There many types of cutis laxa. The NORD report referenced in the link at the right lists twenty different subtypes, and although some of these listings are different names for the same condition, there are still many forms of CL. Overall, however, all of these subtypes fall into two main groups: 1) cutis laxa with involvement of multiple organs, and 2) cutis laxa that causes growth delay and that affects the central nervous system by causing intellectual disabilities, seizures, brain abnormalities, and other problems (1).
In addition, CL appears to occur in all ethnic groups. In spite of this fact and the number of different CL subtypes, the condition as a whole is so rare that even when all forms are bundled under the name cutis laxa, prevalence is still estimated at only 1 person per 4 million (2).
Some forms of CL are inherited and others are acquired. Of the inherited forms, some are recessive, meaning that both parents contributed a mutated gene. Others are dominant, meaning that one parent contributed a copy of a mutated gene or that the mutation occured spontaneously.
Clinical information
Cutis laxa type 2 has two subtypes: 2A and 2B. There is considerable overlap between these two subtypes, and both are members of the second main category noted above (CL with growth delay and central nervous system involvement). The medical problems that occur in CL type 2 are as follows:
- Skin that is loose and sags (typically present at birth; may improve with time)
- Loose/lax joints and ligaments
- Hypotonia (low muscle tone; "floppiness")
- Intellectual disability (may be more likely to be mild in type 2B patients)
- Delays in motor development
- Failure to thrive
- Microcephaly (small head)
- Large anterior fontanel (soft spot on the top of the head)
- Osteopenia (low bone mineral density, but not low enough to be osteoporosis)
Common problems in Cutis Laxa, types 2A and 2B
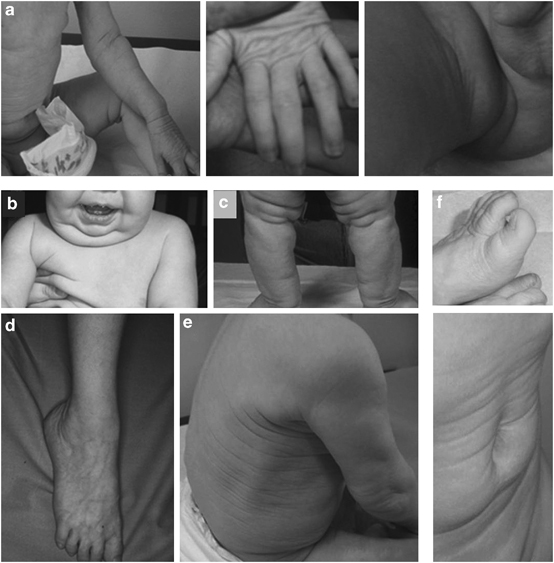
In addition, CL type 2A and 2B patients often share a similar facial appearance, with characteristics as follows:
- Long philtrum (the groove below the nose)
- Broad nasal bridge
- Large forehead
- Wideset eyes
- Anterverted nares (upturned nose)
- Low-set ears
Facial features in cutis laxa, types 2A and 2B
The photograph at the top right of this page shows a young child with cutis laxa type 2. Her eyes are wideset and they slant downward. Her philtrum (groove below her nose) is long, and her nose is slightly upturned. Her skin is also sagging slightly; this abnormality is most obvious in her lower cheeks and eyelids.
Mutations and severity groups in cutis laxa type 2
Cutis laxa type 2 can be caused by mutations in different genes, including ATP6V0A2 (type 2A) and PYCR1 (type 2B). It is likely that other genes will be associated with CL2 in the future. Both subtypes are inherited in an autosomal recessive manner, meaning that both parents must contribute a mutated copy of the gene. Cutis laxa 2A (mutations in ATP6V0A2) can be mild to severe, and it includes conditions called wrinkly skin syndrome and Debré-type cutis laxa. There are no correlations between the precise mutation in ATP6V0A2 and disease severity. Wrinkly skin syndrome is a milder form, while Debré-type cutis laxa is severe.
In Debré-type cutis laxa, patients tend to have intellectual disabilities with delayed language development and neurodegeneration. The most striking feature of this subtype is cobblestone-like dysgenesis, a condition in which the surface of the brain is bumpy. This abnormality is caused by "misplaced neurons and glial cells that have migrated beyond the normal surface boundaries of the brain" (3). Cobblestone-like dysgenesis has not been reported in other forms of cutis laxa. Debré-type patients also tend to develop seizures that begin by their tenth birthdays. They may also have relatively severe myopia.
Patients with wrinkly skin syndrome (again, ATP6V0A2) tend to have milder cognitive disabilities without neurodegeneration. Skin characteristics in this group are also less severe, though they, like Debré-type patients, typically develop a prematurely aged appearance in in their faces, hands, and other areas in their early teens or sooner.
The clinical features and severity of cutis laxa caused by PYCR1 mutations vary. In addition to the problems noted for type 2A patients, these patients may have intrauterine growth retardation (less likely in ATP6V0A2/type 2A patients; 4) and may also develop scoliosis. Individuals originally diagnosed with gerodermia osteodysplastica, wrinkled skin syndrome and De Barsy syndrome have been found to carry a PYCR1 gene mutation
In some cases, CL2 may be diagnosed via gene sequencing. The links at the right provides a search for labs that test for cutis laxa type 2 (A and/or B) and all types of cutis laxa. However, as noted above, researchers do not currently have a complete list of genes associated with this disease. Thus, a patient with CL type 2 may not test positive in a gene sequencing test.
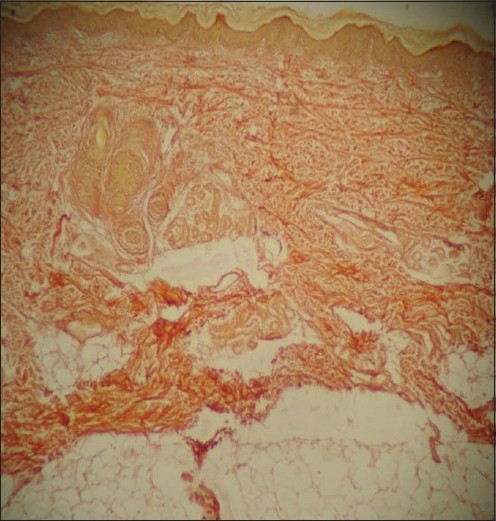
Skin biopsies are another way to gather information suggestive of a diagnosis of CL2. Typical features include severe elastin abnormalities (absence of/very few elastic fibers in the upper dermis and/or irregular or "fuzzy-looking fibers that are short and/or broken). Biopsies also show irregularly shaped elastic fibrils of mid-dermis that may look immature (1, 5). The extracellular matrix may also appear to be abnormal. The micrograph at the right shows a skin biopsy from a patient with cutis laxa type 1. The skin sample had been stain with Verhoeff's stain, which colors elastic fibers brown to blue-black. Note that no staining of this color is evident in the photo (not all patients will have a complete absence of staining with this technique).
Cutis laxa type 2 is most similar to gerodermia osteodysplastica (GO) and de Barsy syndrome. There is considerable overlap in the phenotype (observable characteristics) of these conditions, and GO is sometimes categorized as CL type 2. Sequencing may be able to distinguish these conditions, but genes associated with each condition have not been found, and it is likely that genes other that genes other than ATP6V0A2 and PYCR1 will eventually associated with cutis laxa type 2. Briefly, patients with all three conditions have lax and/or sagging skin. Skin abnormalities are generalized in CL2 patients, whereas in GO, skin abnormalities tend to be localized to the hands and feet, and to the stomach when he patient is seated. GO patients also do not tend to have intellectual disabilities or microcephaly. De Barsy syndrome patients may develop cataracts. They generally have intellectual disabilities. Complicating the picture is the fact that patients with mutations in PYCR1 have been diagnosed with each of these three diseases. In summary, distinguishing these diseases may be very difficult.
Cutis laxa type 2 is superficially similar to cutis laxa, type 1A. The two conditions share many other common features, including loose skin, hernias, lax joints, and hypotonia. CL type 1A is characterized by loose skin that does not improve with age, and by systemic involvement (most commonly tortuous blood vessels, narrowed blood vessles, and aneurysms. Patients may also have lax joints and long, slender fingers). CL type 1 can be severe when heart and lung problems (including emphysema) are present. However, it should be noted that emphysema is rare to non-existent in patients with type 2 cutis laxa. While the presence of emphysema may rule out CL type 2, its absence does not carry definitive diagnostic value for CL type 2, as not all CL type 1 patients have emphysema. The conditions may be best distinguished by gene sequencing.
Cutis laxa may be confused with Ehlers-Danlos syndrome because of joint laxity and skin abnormalities. However, the skin in Ehlers-Danlos patients typically springs back into place after being stretched, while the skin of cutis laxa patients does not. Hutchinson-Gilford progeria syndrome (HGPS) is also superficially similar to cutis laxa, but CL patients do not have the characterisic faces of HGPS patients, nor do CL patients generally experience hair loss in the way that HGPS patients do.
Other conditions in the differential diagnosis for CL type 2 are described in the Gene Review linked to at the right and in reference 1. In addition, interested readers may wish to consult the review of cutis laxa that is noted in reference 6.
References
- 1. Gardeitchik T et al. (2014) Clinical and biochemical features guiding the diagnostics in neurometabolic cutis laxa. Eur J Hum Gen 22: 888-895. Full text on PubMed.
- 2. Van Maldergem L & Loeys B (2009) FBLN5-Related Cutis Laxa. Updated August 16, 2018. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 3. Chang B et al. (2005) Polymicrogyria Overview. Updated August 16, 2018. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 4. Fischer B et al. (2012) Further characterization of ATP6V0A2-related autosomal recessive cutis laxa. Hum Genet 131(11):1761-1763. Abstract on PubMed. Full text from authors.
- 5. Tuysuz B et al. (2003) Congential cutis laxa syndrome: type II autosomal recessive inheritance. Turk J Pediatr 45:265-268. Full text from publisher.
- 6. Mohamed M et al. (2011) Metabolic cutis laxa syndromes. J Inherit Metab Dis 34(4):907-916. Full text on PubMed.
- 7. Choudhary SV et al. (2011) Congenital cutis laxa with rectal and uterovaginal prolapse. Ind J Derm Ven Lep 77(3):321-324. Full text from publisher.