Hyper IgM Syndromes (HIGM)
Hyper IgM syndrome is a group of very similar conditions that cause severe immunodeficiency, or a biological defect resulting in poor protection from infections.
There are many types of immunodeficiencies. They all result from problems with the immune system, and are categorized in two groups: primary and secondary. In primary immunodeficiencies, innate problems in the immune system are the direct cause of disease. Most of these conditions are caused by inherited genetic defects. There are over 250 primary immunodeficiencies; HIGM is one of them. Secondary immunodeficiencies occur as a result of another problem, such as HIV infection or as a side-effect of a drug.
People with HIGM cannot make antibodies, which fight many types of infections. This situation makes them susceptible to infections caused by organisms that do not cause sickness in unaffected people. In particular, three types of antibodies are affected in HIGM. They are called IgG, IgA, and IgE. These antibodies are made in white blood cells called B cells, when the cells become activated as a result of infection. B cells that have not been activated produce IgM antibodies, and activation prompts them to switch from IgM to IgG or another type of antibody such as IgA.
IgG is the most common type of antibody, and it has many functions in fighting infection. IgM cannot perform many of these functions, in part because it is so large and cannot diffuse into tissues. This is one reason for why HIGM is a serious disease.
Clinical information
The clinical features of the different HIGMs are generally similar. In most cases, recurrent infections before the second birthday (often before age 1), although a small number of patients are not affected until later.
A baby does not produce IgG type antibodies before birth. After birth, production begins and increases slowly with time. Fortunately, IgG antibodies are passed from mother to child through the placenta, and by the end of a full-term pregnancy, most infants have levels of IgG equal to their mothers. For this reason, most infants with HIGM are healthy at birth and for weeks or months afterwards --- until maternal antibodies begin to wane. In general, maternal IgG type antibodies fall below protective levels around 6-12 months of age. At this point, infants who cannot produce IgG by themselves become susceptible to infections. Antibodies of the IgA type do not pass through the placenta, but are present in breast milk. They help protect against infections of the gastrointestinal (GI) tract. Antibodies in breast milk do not leave the GI tract.
Antibodies of the IgA type do not pass through the placenta, but are present in breast milk. They help protect against infections of the gastrointestinal (GI) tract. Antibodies in breast milk do not leave the GI tract and cannot provide general protection against infection.
As an example, an infant with HIGM may develop what appears to be a simple cold. In some cases, the same condition affects another family member who recovers quickly. The HIGM patient, alternatively, may deteriorate rather than recover. For example, he may become listless and breath rapidly. Fever may be present. If a lower respiratory infection such as pneumonia is present, a doctor may hear abnormal lung sounds through a stethoscope.
Common clinical features of HIGM are listed below.
- Frequent infections, especially respiratory infections
- Infections tend to begin before age 1
- Diarrhea, which may be chronic
- Pneumonia, often before age 2
- Frequent ear infections
- Opportunistic infections
- Mouth ulcers
- High or normal serum IgM
- Low serum IgG
- Low serum IgA
- Neutropenia
Common clinical features of Hyper IgM syndrome
HIGM patients with certain gene mutations (see below) are also susceptible to cancers, that appear to develop later in life (1, 2). Liver disease is a serious complication of HIGM and a frequent cause of death (3).
Causes
Hyper IgM syndrome can be caused by mutations in one of several genes. The most common cause of the syndrome is a mutation in CD40L (also called CD154 or TNFSF5; reviewed in reference 4), CD40L is located on the X-chromosome, meaning that HIGM is an X-linked disorder. This term refers to the fact that females have two X chromosomes and males have one. This is why X-linked disorders tend to affect males more seriously than females --- if a girl inherits a faulty copy of the chromosome, she will likely have a normal copy that can compensate for the faulty one. It is estimated that 70% of cases of HIGM are due to mutations in CD40L (5).
Thus, in boys with HIGM, the mutated gene has been passed from their mothers. Fathers pass Y chromosomes to their sons and therefore cannot transmit a damaged X-chromosome to a boy. Mothers can also pass the mutated gene to daughters, but as noted above, their second X chromosome compensates for the mutation. Girls do not have signs of HIGM beyond decreased levels of the CD40L molecule. Female carriers still produce enough CD40L to create antibodies. In very rare cases, girls with mutations in CD40L shows signs of immunodeficiency, such as when the girl's second X chromosome also has abnormalities (6, 7).
This type of HIGM has only been identified in a small number of patients (for examples, see references 8-12) and is clinically indistinguishable from X-linked HIGM. This is because protein made by the mutated gene, CD40, interacts with the protein made by the CD40L gene. When either is affected, the same clinical features result. The primary difference between types 1 and 3 is that girls may be affected by type 3. This is because type 3 HIGM is autosomal recessive. The term autosomal recessive means that the disorder is passed on when both parents contribute a copy of the mutated gene to their child.
This form of HIGM shares many clinical features with types 1 and 3. Most obviously, blood levels of IgG and IgA are low, and IgM levels are normal or high, leading to recurring bacterial infections, especially in the lungs and the rest of the respiratory tract. Gastrointestinal infections are also common. However, patients with mutations in AICDA do not appear to be susceptible to opportunistic infections and cancers. The lack of cancers may be due to the relatively low number of patients identified to date, however, rather than to biological factors. Roughly 125 people with HIGM caused by AICDA mutations have been reported in the literature (13).
HIGM may also be caused by mutations in the genes UNG (type 5 HIGM) and PIK3R1 (unnumbered subtype). Very few patients with mutations in these genes have been identified. For example, a recent paper noted that only 16 cases of PIK3R1-HIGM have been described in the literature (14). Type 4 HIGM is not associated with a gene as of yet, but it appears to be a milder form of disease (15). As with other forms of HIGM, in patients with these subtypes, IgM levels are normal or high and IgA and/or IgG levels are low. Again, this situation leads to recurring bacterial infections, especially in the lungs and the rest of the respiratory tract. Opportunistic infections have been reported in 4 PIK3R1 cases reported to date (16-18).
Diagnosis and Testing
The Immune Deficiency Foundation notes that hyper IgM syndrome should be suspected in any infant with low severe recurring respiratory infections or an opportunistic infection, combined with low or absent IgG and normal or high levels of IgM. Testing may involve flow cytometry to look for the absence of CD40L on cells and/or genetic sequencing (see links at right).
As noted above, patients tend to develop respiratory infections at a very young age. Although a large majority of cases of HIGM are X-linked and occur in boys, girls may also be affected. Thus, the diagnosis should not be excluded because the patient is a girl. It is also important to remember that HIGM patients with low levels of IgM have been identified (2, 19). Normal levels of IgG have also been found (2, 20, 21). Diagnosis was confirmed in these patients by genetic sequencing. Thus, as is commonly the case, with rare conditions, diagnosis may be tricky, and specific HIGM testing may be warranted in patients with an infection profile fitting HIGM. Note also that ataxia-telangiectasia patients may resemble HIGM patients (see below).
The links on the right side of this page provide information about testing labs around the world.
Treatment
Treatment of HIGM requires a team of specialists (e.g. pediatricians, immunologists, nutritionists, pulmonologists, hematologists, and others, depending on an individual patient's needs). These options were discussed in a recent review (3). They include regular adminisration of antibodies to reduce the number of infections, as well as regular doses of antibiotics to fight infections that do occur. According to the Immune Deficiency Foundation, patients with types 1 or 3 HIGM should not be given live-virus vaccines, due to the risk that disease will result.
Bone marrow stem-cell transplantation has been used to treat HIGM caused by mutations in CD40L and CD40 (22-25), but it is not always successful: a study of transplant outcomes in 38 patients found a 68% survival rate, with 20 responding to transplant well enough to be described as cured and not needing antibody replacement therapy (26). This same study noted the following: "Of the 6 patients with preexisting hepatic disease receiving fludarabine/melphalan (low intensity) conditioning regimens there were 3 survivors, compared with 8 of 12 with preexisting hepatic disease who received busulphan/cyclophosphamide-containing conditioning regimens."
Differential Diagnosis
The hyper IgM syndromes are rare, even among rare diseases. Diagnosis can be difficult as a result, as many clinicians may not even know of their existence. A number of other conditions resemble HIGM and are described below.
Ataxia-Telangiectasia (A-T). A-T is a DNA repair disorder that causes neurological problems and immunodeficiency. The latter problem is due to a patient's inability to repair certain break in DNA that are necessary for making antibodies. Like people with HIGM, A-T patients often have low levels of IgA and/or IgG in their blood along with normal or high levels of IgM. A-T patients may develop pneumonias and other respiratory infections at very young ages. Unlike HIGM patients, they are not apparently susceptible to opportunistic infections.
One sign that may help distinguish the two conditions is that A-T patients tend to have wobbly, unsteady gaits from the time that they learn to walk. As time passes, they develop a variety of neurological problems that get progressively worse. These problems include development of ataxia, which is a loss of coordination. The wobbly walk is the first sign of ataxia. Patients may also sway when sitting or standing. Other forms of ataxia include problems with the hands (difficulty using utensils or buttoning clothes, for example). Dysmetria is difficulty moving a pointed finger to an object and back to another place, such as touching the tip of the nose. A-T patients lose deep tendon reflexes (such as ankle or knee jerks). HIGM patients do not generally have neurological problems, but they can occur in a minority of patients who develop infections of the central nervous system. Based on clinical signs, these patients may be almost indistinguishable from A-T patients.
A-T patients often have dilated blood vessels in their eyes or on their skin. These blood vessels are called telangiectases. They may not appear until a child is 5 or 6 years old. HIGM patients do not develop telangiectases, but they do develop conjunctitivis of the eye, which may look similar to telangiectases. Finally, A-T patients have high blood levels of a substance called AFP. HIGM patients do not have this feature.
Agammaglobulinemias (XLA and XRA). The agammaglobulinemias are conditions causing lack of certain antibodies. XLA refers to X-linked agammaglobulinemia, while ARA refers to autosomal recessive agammaglobulinemia. Regardless of the cause, patients make very few mature B cells, rendering them poorly able to produce IgG, IgA, IgE, and IgM. Like HIGM patients, XLA and XRA patients are prone to developing infections such as pneumonia and other lung infections (e.g. bronchitis), ear infections, sinusitis, and gastrointestinal infectins. Also like HIGM patients, infections tend to develop after protection from maternally acquired antibodies has waned. Low levels of IgM and few B cells can distinguish agammaglobulinemia from HIGM. Another feature that may distinguish the two conditions is the size of the tonsils and/or the lymph nodes. Both are largely composed of B lymphocytes. Because XLA and ARA patients make few B lymphocytes, their tonsils and lymph nodes tend to be very small. HIGM patients may develop enlarged tonsils and/or lymph nodes.
HIV infection. HIV testing should be performed in any child with pneumonia caused by Pneumocystis jeroveci (formerly called Pneumocystis carini/PCP pneumonia). P. jeroveci is a common fungus that rarely causes infection in healthy people. It does cause infection in people with weakened immune systems.
Common variable immunodeficiency (CVID). CVID is a condition that features low levels of antibodies, especially IgG and IgA. Roughly half of patients also have defiencies of IgM (27). CVID is relatively common among the immunodeficiencies, with a prevalence as high as 1 person in 10,000 (27) to 1 in 25,000 (28). The clinical features of CVID are similar to those of HIGM and the other immunodeficiencies described here, and include susceptibility to respiratory infections including pneumonia and sinusitis. CVID can develop in adults, and a difference between it and HIGM is that CVID patients may have decreased numbers of total T cells or decreased T-cell function.
Other conditions in the differential diagnosis of hyper IgM syndrome may be found in reference 15.
References
- 1. Filipovich L & Gross T (2004) Immunodeficiency and cancer. In: Abeloff M, Armitage J, et al., eds. Clinical Oncology (3rd edition) London, UK: Elsevier/Churchill Livingstone; pp. 287-98. Entry on amazon.com.
- 2. Winkelstein JA et al. (2003) The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 82(6):373-384. Abstract on Pubmed. Full text on ResearchGate.
- 3. Qamar N & Fuleihan RL (2014) The hyper IgM syndromes. Clin Rev Allergy Immunol 46(2):120-130. Abstract on PubMed.
- 4. Levy J et al. (1997) Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr 131(1 Pt 1):47-54. Abstract on PubMed.
- 5. Davies EG & Thrasher AJ (2010) Update on the hyper immunoglobulin M syndromes. Br J Haematol 149(2):167-180. Full text on PubMed.
- 6. de Saint Basile G et al. (1999) CD40 ligand expression deficiency in a female carrier of the X-linked hyper-IgM syndrome as a result of X chromosome lyonization. Eur J Immunol 29(1):367-373. Abstract on PubMed.
- 7. Imai K et al. (2006) Female hyper IgM syndrome type 1 with a chromosomal translocation disrupting CD40LG. Biochim Biophys Acta 1762(3):335-340. Full text available from publisher.
- 8. Al-Saud BK et al. (2013) Clinical, immunological, and molecular characterization of hyper-IgM syndrome due to CD40 deficiency in eleven patients. J Clin Immunol 33(8):1325-1335. Abstract on PubMed.
- 9. Ferrari S et al. (2001) Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci USA 98(22):12614-12619. Full text on PubMed.
- 10. Kutukculer N et al. (2003) Disseminated cryptosporidium infection in an infant with hyper-IgM syndrome caused by CD40 deficiency. J Pediatr 142(2):194-196. Abstract on PubMed.
- 11. Kutukculer N et al. (2003) Outcome of hematopoietic stem cell transplantation in hyper-IgM syndrome caused by CD40 deficiency. J Pediatr 143(1):194-196. Abstract on PubMed.
- 12. Revy P et al. (2000) Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2). Cell 102(5):565-575. Full text available from publisher.
- 13. Trotta L et al. (2016) Enrichment of rare variants in population isolates: single AICDA mutation responsible for hyper-IgM syndrome type 2 in Finland. Eur J Hum Genet 24(10):1473-1478. Full text available from publisher.
- 14. Petrovski S et al. (2016) Dominant splice site mutations in PIK3R1 cause hyper IgM syndrome, lymphadenopathy and short stature. J Clin Immunol 36(5):462-471. Full text available from author.
- 15. Johnson J et al. (2007) X-Linked Hyper IgM Syndrome. Updated January 24, 2014. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2017. Full text.
- 16. Deau MC et al. (2014) A human immunodeficiency caused by mutations in the 574 PIK3R1 gene. J Clin Invest 124(9):3923-3928. Full text on PubMed.
- 17. Lougaris V et al (2015) Altered germinal center reaction and abnormal B cell peripheral maturation in PI3KR1-mutated patients presenting with HIGM-like phenotype. Clin Immunol 159(1):33-36. Abstract on PubMed.
- 18. Lucas V et al (2014) Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med 211(13):2537-2547. Full text on PubMed.
- 19. Heinold A et al. (2010) Pitfalls of "hyper"-IgM syndrome: a new CD40 ligand mutation in the presence of low IgM levels. A case report and a critical review of the literature. Infection 38(6):491-496. Abstract on Pubmed.
- 20. Buchbinder D et al. (2012) X-linked hyper IgM syndrome: a novel sequence variant associated with an atypical mild phenotype. J Pediatr Hematol Oncol 34(5):e212-214. doi: 10.1097/MPH.0b013e318241fa1b. Abstract on Pubmed.
- 21. Leone V et al. (2002) Elective bone marrow transplantation in a child with X-linked hyper-IgM syndrome presenting with acute respiratory distress syndrome. Bone Marrow Transplant 30(1):49-52. Full text available from publisher.
- 22. Thomas C et al. (1995) Brief report: correction of X-linked hyper-IgM syndrome by allogeneic bone marrow transplantation. N Engl J Med 333(7):426-429. Full text available from publisher.
- 23. Scholl PR et al. (1998) Correction of neutropenia and hypogammaglobulinemia in X-linked hyper-IgM syndrome by allogeneic bone marrow transplantation. Bone Marrow Transplant 22:1215-1218. Full text available from publisher.
- 24. Mazzolari E et al. (2007) First report of successful stem cell transplantation in a child with CD40 deficiency. Bone Marrow Transplant 40(3):279-281 Full text available from publisher.
- 25. Al-Dhekri H et al (2012) Successful outcome in two patients with CD40 deficiency treated with allo-geneic HCST. Clin Immunol 143(1):96-98. Abstract on PubMed.
- 26. Gennery AR et al. (2004) Treatment of CD40ligand deficiency by haemopoietic stem cell transplantation: a survey of the European experience, 1993–2002. Blood 103(3):1152-1157. Full text available from publisher.
- 27. Hammarstrom L & Smith CIE (1999) Genetic approach to common variable immunodeficiency and IgA deficiency. In: Ochs, HD, Smith, CIE, & Puck JM (eds.) Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. New York: Oxford University Press; pp. 250-262. Entry on amazon.com.
- 28. Hammarström L et al. (2000) Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID). Clin Exp Immunol 120(2):225-231. Full text on Pubmed.
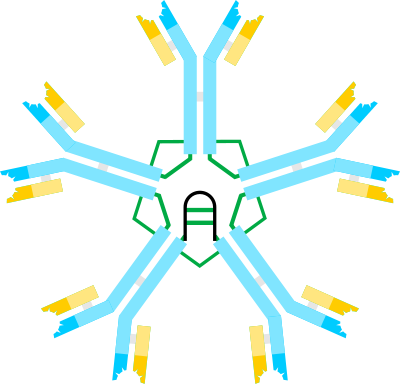
- 29. Fijałkowski J (2006) Schematic drawing of an IgM molecule. Image from the Wikimedia Commons.
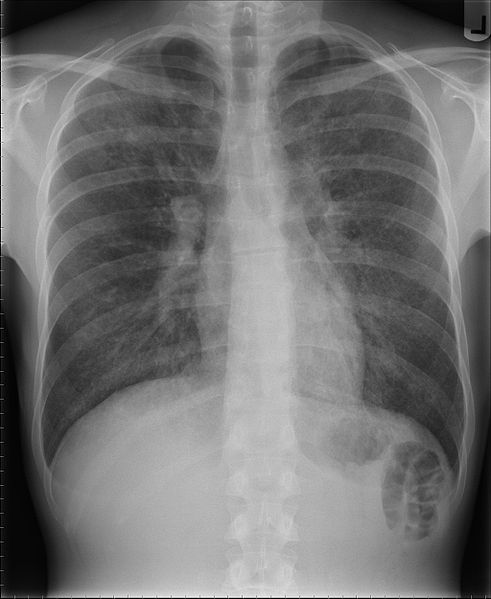
- 30. White, G (2005) X-ray of patient with pneumonia. Image from the Wikimedia Commons.
- 31. Samir, Wikimedia Commons user (2007) X-ray of patient with pneumonia. Image from the Wikimedia Commons.