Marfan syndrome (MFS)
Marfan syndrome (MFS) is a connective tissue disorder. Connective tissue helps hold the body together and helps it develop properly. It includes (but is not limited to) adipose tissue, tendons, ligaments, cartilage, bones, and even blood. Connective tissue disorders affect one or more types of these tissues. For example, collagen is affected in Ehlers-Danlos syndrome. Collagen is an abundant structural protein found in muscles, tendons, skin and bones; it holds the body together and gives it strength.
Marfan syndrome affects many types of connective tissue. This is because the gene that is mutated in MFS affects systems throughout the body. People with Marfan syndrome can have problems with their eyes, their hearts and blood vessels, their joints, skin, lungs, and other organs. In some people, MFS is life-threatening. Others may have mild symptoms. Fortunately, medical advances in recent decades have made it possible for many people with this condition to lead normal lives with normal life expectancy.
Marfan syndrome occurs in both sexes and in all races and ethnic groups. Researchers believe that it affects 1 American in 5,000 (1). This figure means that there are roughly 62,000 people with MFS in the United States.
Clinical information
The most striking feature of Marfan syndrome is that affected individuals tend to be slender and very tall. A study of height in MFS found that the mean height of people with Marfan syndrome was above the 95th percentile for the general population by their third birthdays (2).
However, some people with MFS are not tall compared to the general population, and a very small percentage of patients may even at the population average or slightly below it. This fact means that lack of tall stature should not be used to rule out Marfan syndrome. In some cases, comparing with parental height may be useful. A person suspected of having MFS but who is not tall in the general population may still be tall when compared to unaffected members of his or her family.
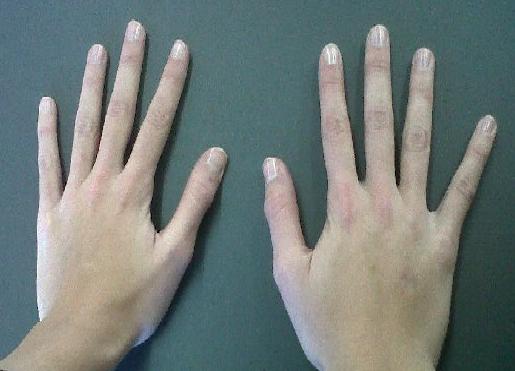
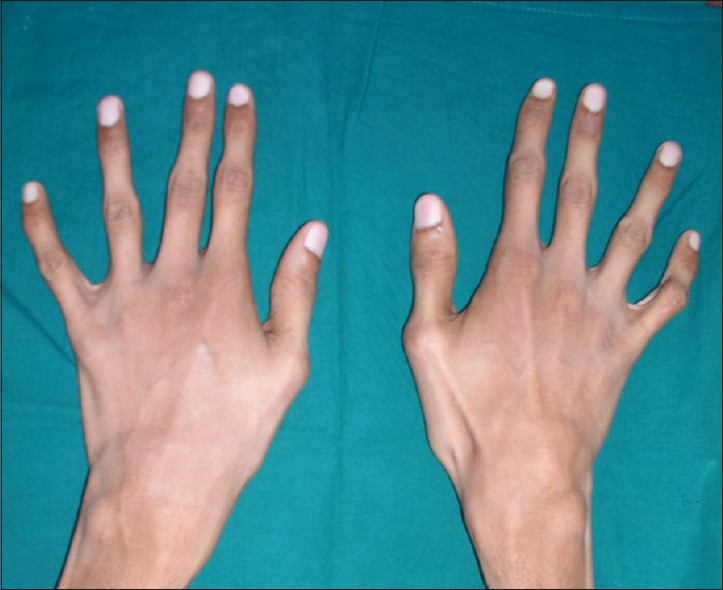
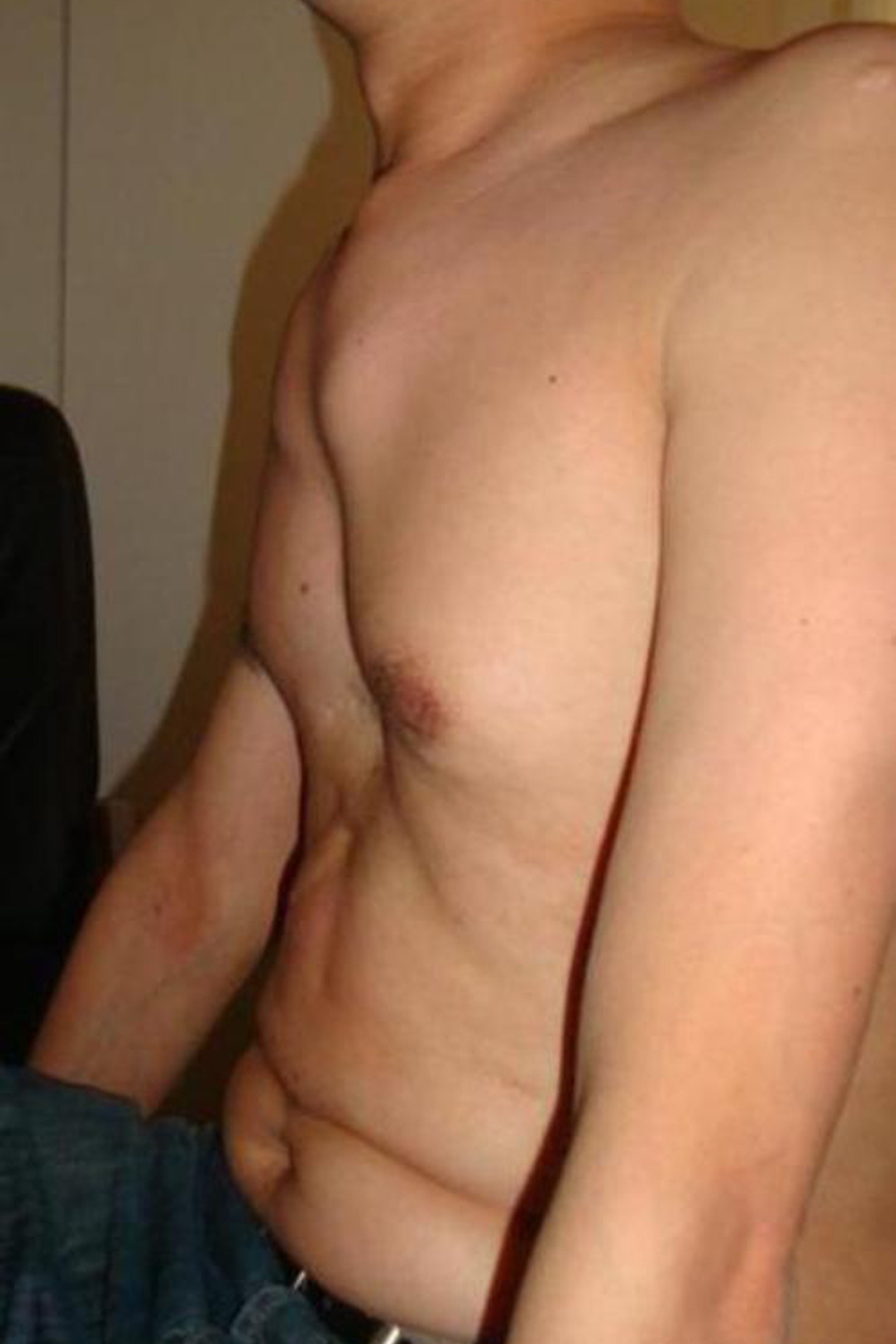
People with Marfan syndrome tend to have what is called a Marfanoid habitus. This term refers to tall stature, long limbs (arm span is usually greater than height), long fingers, crowded teeth, a high-arched palate, and loose/lax joints. See the photos on this page for examples.
Marfan syndrome affects a variety of body systems. In particular, cardiac problems are of note in this population, as are skeletal abnormalities (especially the chest and hands), and problems with the eyes and vision. Marfan syndrome does not affect intelligence. The following are common problems in this syndrome (this list is not exhaustive):
- Enlargement of the root of the aorta (a portion of the aorta)
- Dissection of the aorta (tears in the internal wall of the aorta)
- Mitral valve prolapse (valve does not close properly)
- Other cardiovascular problems
- Joint laxity/hypermobility
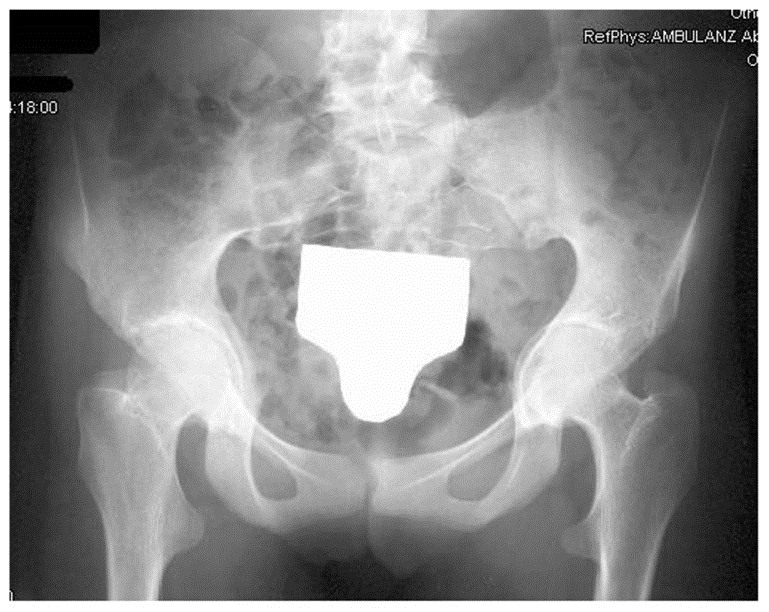
- Acetabular protrusion (an abnormality of the acetabulum of the hip); see photo
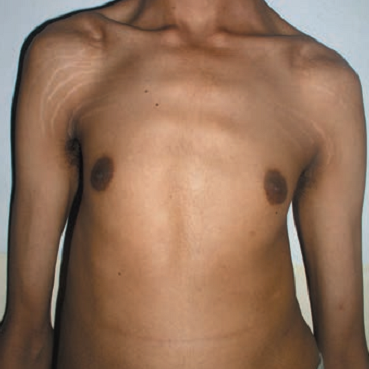
- Pectus excavatum (sunken chest) or carinatum (pigeon chest)
- Scoliosis or other spinal curvature abnormalities
- Dural ectasia (bulging of the spinal column lining)
- Flat feet
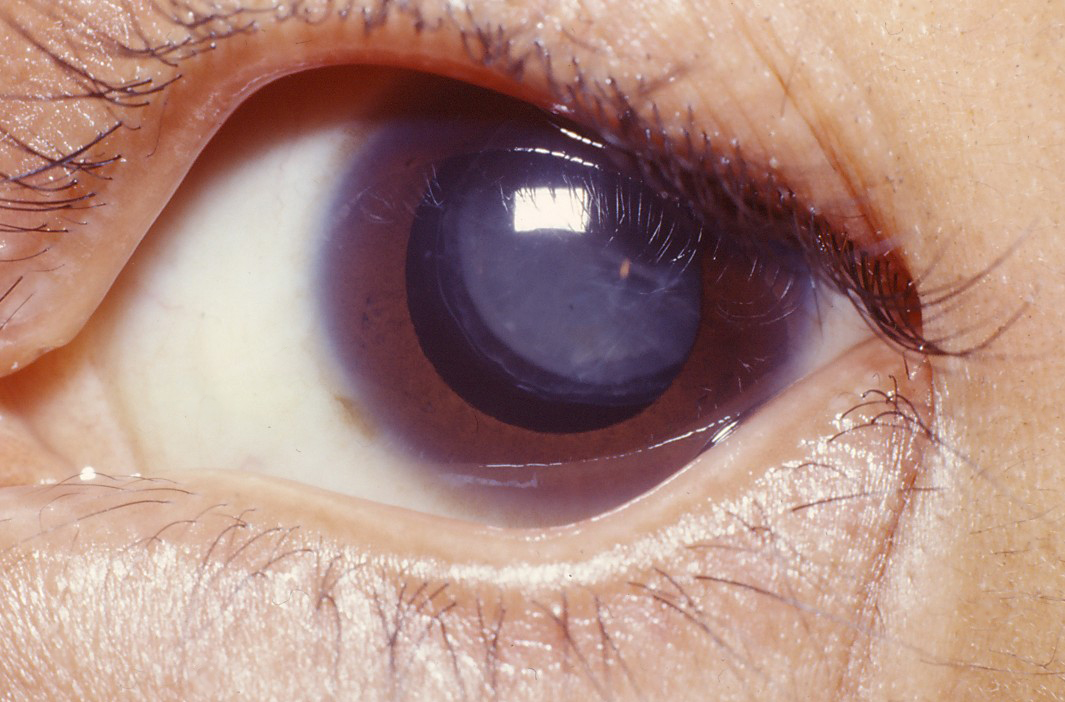
- Dislocation of the eye lens (ectopia lentis); see photo
- Nearsightedness that may be severe
- Cataracts
- Stretch marks on the skin
- An armspan that is greater than height
Common clinical features of Marfan syndrome
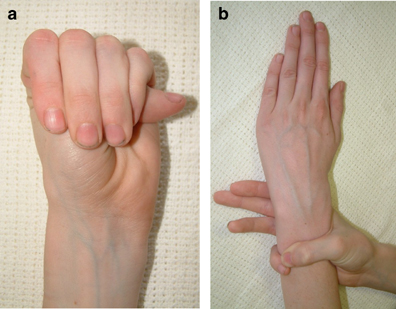
Two important clinical features of Marfan syndrome are the wrist and thumb signs (see photos below). The presence of these is an important indicator for MFS, and testing for them is simple. A thumb sign means that when a person makes a fist over her thumb, the thumb juts out from the fingers, (see photo below). A wrist sign means that a person's pinky finger and thumb overlap when he wraps his fingers around his wrist (see photo below). These signs are part of the diagnostic criteria for Marfan syndrome (see Diagnosis and Testing, below).
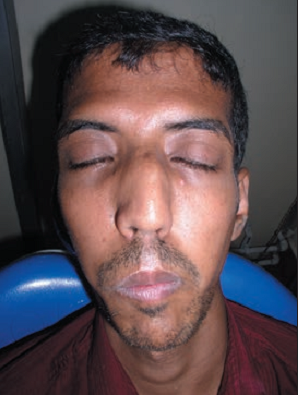
Many people with Marfan syndrome have a characteristic facial appearance (see photos on this page). Many have a long face that is narrow, underdeveloped cheek bones, a small lower jaw that recedes, and a highly arched palate. Some people also have downslanting palpebral fissures, or eyes that appear to slant downward from the center outward. These features can help with diagnosis, but they do not occur in all patients.
Diagnosis and Testing
The Marfan Foundation has a free online tool that can help with diagnosing Marfan syndrome.
Marfan syndrome is an autosomal dominant disorder. This term means that only one copy of a mutated gene is needed to cause the disorder. Most patients have an affected parent: in our literature survey of nearly 2,000 people diagnosed with MFS, we found that 53% were said to have an affected family member. This means that sporadic cases also occur in a significant number of patients. In a sporadic case, the mutation occurs spontaneously, typically with no known reason. Marfan syndrome is caused by mutations in the gene fibrillin 1 (FBN1). For a list of labs that test for mutations in this gene, see the link at right.
Diagnostic criteria for MFS have been established. They include major and minor criteria and a formal system to help clinicians make decisions. This system is called the revised Ghent nosology (3). The following are major criteria that indicate a diagnosis of Marfan syndrome. This information has been adapted from reference 3.
Important: in some cases, a diagnosis of MFS can only be made in the absence of features of related syndromes, such as Sphrintzen-Goldberg syndrome (SGS), Loeys-Dietz syndrome (LDS), and the vascular form of Ehlers-Danlos syndrome (vEDS). See Differential Diagnosis, below, for details about these conditions.
No family history of Marfan syndrome and no signs of SGS, LDS, or vEDS:
- Aortic diameter >2 standard deviations above mean AND ectopia lentis
- Aortic diameter >2 standard deviations above mean AND Systemic Score >7 (see table below)
No family history of Marfan syndrome:
- Aortic diameter >2 standard deviations above mean AND mutation in FBN1
- Ectopia lentis AND mutation in FBN1 AND enlarted aortic diameter
A family history of Marfan syndrome:
- Ectopia lentis AND family history
- Systemic Score >7 (see table below) and family history
- Aortic diameter >2 standard deviations above mean (age >20) or >3 standard deviations (age <20) AND family history
The Systemic Score
The criteria in the table below assist with a diagnosis (3). The score from the table is called the systemic score. Higher scores make a diagnosis of Marfan syndrome more likely. The maximum possible score in this system is 20 points (points in parentheses do not count toward the maximum). A score of at least 7 indicates that a person has systemic connective tissue abnormalities.
Clinical feature |
|
Score |
Wrist AND thumb sign: 3 points (Wrist OR thumb sign: 1 point) Pectus carinatum: 2 points (Pectus excavatum or chest asymmetry: 1 point) Hindfoot deformity: 2 points (Flat feet: 1 point) Pneumothorax: 2 points Dural ectasia: 2 points Acetabular protrusion (see photo): 2 points Pectus excavatum or chest asymmetry: 1 point Body measurements: reduced upper segment to lower segment ratio AND
Scoliosis or thoracolumbar kyphosis: 1 point At least 3 of the following facial features: 1 point Dolichocephaly (long skull; see photos) Sunken eyes (enophthalmos) Downslanting palpebral fissures Receding lower jaw (retrognathia) Underdeveloped cheekbones (malar hypoplasia) Stretch marks on the skin: 1 point Myopia (nearsightedness); >3 diopters: 1 point Any type of mitral valve prolapse: 1 point |
Differential Diagnosis
A number of conditions are in the differential diagnosis for Marfan syndrome. This section divides them into two groups: those that are generally associated with mutations in FBN1, mutations in which also cause Marfan syndrome, and those that are not generally associated with mutations in FBN1. The list here is not exhaustive, but we believe that it captures conditions most likely to be confused with MFS.
I. Conditions caused by mutations in FBN1
The conditions described in this section should only be considered in people who are over age 20. This is because, unlike MFS, the clinical features of these conditions are not progressive. The features of Marfan syndrome may develop slowly as a person ages, and may not become fully apparent until the late teens or age 20 (4). Thus, it is important not to make a premature diagnosis of a non-progressive condition.
The MASS phenotype is a condition that overlaps with Marfan syndrome. The letters MASS stand for Mitral valve, Aorta, Skin and Skeletal features. The MASS phenotype is autosomal dominant. Mitral valve prolapse is one of its important features. Aortic root enlargement occurs, but it may be borderline and it does not get worse with time or progress to being an aneurysm. People with the MASS phenotype may also have myopia, scoliosis, pectus deformities of the chest, lax joints, and skin stretch marks. Importantly, they do NOT have ectopia lentis/lens dislocation.
The two key differences between MASS and MFS are a more benign form of aortic root enlargement and lack of lens dislocation. Because aortic root enlargement does not progress in the MASS phenotype, this condition cannot be diagnosed until a person is older than age 20 (by which time progression of the aortic enlargement should have occured if the person had MFS).
Mitral valve prolapse syndrome (MVPS). People with MVPS have a prolapsed mitral valve, aortic root enlargement, and a systemic score <5. Aortic root enlargement in this group is always within 2 standard deviations of the mean, meaning that it is not as severe as it is in Marfan syndrome. Individuals with MVPS do not have ectopia lentis/lens dislocations, and it presence precludes a diagnosis of MFS. People with MVPS may also have chest and spinal column doformities and mild arachnodactyly.
Ectopia lentis syndrome (ELS). This condition involves ectopia lentis and skeletal problems. However, aortic root enlargement does not occur. An adult patient with eye and skeletal signs but with no cardiac involvement and no family members with cardiac involvement may be diagnosed with ELS.
The shared gene and similarities between MFS and these conditions imply that they may be member of a Marfan syndrome spectrum.
II. Conditions caused by mutations in other genes
Loeys-Dietz syndrome (LDS). LDS has many clinical features in common with MFS. For example, patients may have enlarged aortic roots or aortic dissections, chest deformities and scoliosis, club feet, arachnodactyly, lax joints, small/receding jaws, and a highly arched palate. Their arteries may be twisted and tortuous and they are prone to aneurysms. However, people with LDS do not have ectopia lentis. LDS patients may also have widely spaced eyes, cleft palate, blue sclerae, and type 1 Chiari malformations (in which a portion of the cerebellum descends out of the skull into the area where the spinal cord is located). Craniosynostosis (premature fusion of the skull bones, causing an abnormal skull shape) is also a feature of LDS. Patients may have skin abnormalities, including easy bruising, translucent skin, and soft velvety skin. These problems are not associated with Marfan syndrome.
There are two forms of LDS: LDS1 and LDS2. People with LDS1 have craniofacial features (craniosynostosis, widely spaced eyes, cleft palate, bifid uvula). People with LDS2 do not have these features, but have the other features noted above (5).
Like Marfan syndrome, LDS is an autosomal dominant disorder. However, LDS is caused by mutations in TGFBR1, TGFBR2, SMAD3, and TGFB2. This fact means that gene sequencing can distinguish it definitively from MFS.
Sphrintzen-Goldberg syndrome (SGS). Like LDS, SGS shares many features with Marfan syndrome. They include having an armspan greater than height, arachnodactyly, chest deformities and scoliosis, and a highly arched palate. A person with SGS may also have an enlarged aortic root, but this problem is rare (it is very common in MFS). Features of SGS that can help distinguish the two conditions include craniosynostosis, Chiari malformations, widely spaced eyes that may protrude from their sockets, and developmental delays.
Like Marfan syndrome, SGS is an autosomal dominant disorder. It is caused by mutations in SKI, and gene sequencing is able to distinguish it definitively from MFS. However, three cases of SGS with mutations in FBN have been reported (6).
Vascular Ehlers-Danlos syndrome (vEDS). Like MFS, EDS is a connective tissue disorder that is relatively common among rare diseases. People with vEDS may have lax joints, flat feet, and long narrow faces. They may also have severe problems such as aneurysms and pneumothorax. Unlike Marfan syndrome patients, however, they have thin, very fragile skin which may heal poorly. Blood vessel fragility is generalized in vEDS, unlike Marfan syndrome.
VEDS is an autosomal dominant disorder. It is caused by mutations in type 3 collagen (COL3A1). This gene is the only one associated with vEDS at this time. This fact means that gene sequencing can distinguish it definitively from MFS.
Weill-Marchesani Syndrome (WMS). The similarities between WMS and Marfan syndrome are primarily ocular and include ectopia lentis and myopia. Cardiac defects are occasionally assocated with WMS, as well, but they are different from those of MFS and include patent ductus arteriosus (a congenital abnormality in which an opening that exists during the fetal period does not close) and pulmonary stenosis (narrowing of the pulmonary artery). People with WMS tend to be short with short/stubby hands and feet. These differences can make WMS easy to distinguish from Marfan syndrome.
WMS is caused by mutations in a number of genes, including ADAMTS10 and LTBP2. It is included in the differential diagnosis of Marfan syndrome because of ocular similarities and because mutations in FBN1 have been found in one family (7).
References
- 1. Jongmans MC et al. (1997). Disorders of fibrillins and microfibrilogenesis: Marfan syndrone, MASS phenotype, contractural arachnodactyly and related conditions. In Principles and Practice of Medical Genetics, 3rd edition (1997), Rimoin DL et al., eds. New York: Churchill Livingstone, pages. ISBN 978-0443048517.
- 2. Erkula G et al. (2002) Growth and maturation in Marfan syndrome. Am J Med Genet 109(2):100-115. Abstract on PubMed.
- 3. Loeys BL et al. (2010). The revised Ghent nosology for the Marfan syndrome. J Med Genet 47(7):476-485. Abstract on PubMed Full text.
- 4. Dietz HC (2001) Marfan Syndrome. Updated February 2, 2017. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 5. Loeys BL & Dietz HC. (2008) Loeys-Dietz Syndrome. Updated March 1, 2018. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 6. Greally MT (2006) Shprintzen-Goldberg Syndrome. Updated June 13, 2013. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 7. Faivre et al. (2003) In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet 40(1):34-36. Full text on PubMed.
- 8. Staufenbiel I et al. (2013) Periodontal conditions in patients with Marfan syndrome - a multicenter case control study. BMC Oral Health 13:59. doi: 10.1186/1472-6831-13-59. Full text on PubMed.
- 9. Randhawa AK et al. (2012) Marfan syndrome: report of two cases with review of literature. Niger J Clin Pract 15(3):364-368. Full text from publisher.
- 10. Photo found in the Wikimedia Commons.
- 11. Dean JC (2007). Marfan syndrome: clinical diagnosis and management. Eur J Hum Gen 15(7):724-733. Full text from publisher.
- 12. Al Kaissi A et al. (2013) Musculo-skeletal abnormalities in patients with Marfan syndrome. Clin Med Insights Arthritis Musculoskelet Disord 6:1-9. doi: 10.4137/CMAMD.S10279 Full text on PubMed.
- 13. Photo by Margreet Hogeweg and found on Flikr.
- 14. Bakali A et al. (2009) Late diagnosis of Marfan syndrome with fatal outcome in a young male patient: a case report. Cass J 2:8827. doi: 10.1186/1757-1626-0002-0000008827. Full text on PubMed.