Noonan Syndrome (NS)
Noonan syndrome, or NS, is a member of a group of conditions called RASopathies. RASopathies often affect growth and development. They share a number of other clinical features include common facial appearances, heart problems, neurlogical problems, and problems with the gastrointestinal tract. Individual RASopathies are rare, but as a group, they are common.
RASopathies are caused by mutations in genes that have roles in a pathway called Ras-MAPK. A pathway is a series of actions that occur in a cell. The actions lead to changes, like cell division or to something being made (a scab or new fat cells) or released (insulin). In some ways, pathways are like Rube Goldberg machines, because one molecule interacts with another one, which causes something else to happen, and so on. Pathways allow different body systems to work with each other. They are found in all processes in living things. Not surprisingly, when one or more pathways do not function properly, medical problems can result.
In the Ras-MAPK pathway, proteins communicate signals from outside a cell to the DNA inside the cell's nucleus. One result is a signal telling the cell to duplicate its DNA and then divide into two cells. If the Ras-MAPK pathway isn't functioning properly, cell division can be affected, such as by being overactive. When cells divide too frequently, tumors and cancer can result. As an example, people with Noonan syndrome have an increased risk for leukemia and other forms of cancer (1).
Other RASopathies include Costello syndrome, Noonan syndrome with multiple lentigines (formally LEOPARD syndrome), neurofibromatosis type 1, cardio-facio-cutaneous (CFC) syndrome, and Legius syndrome.
Clinical information
Noonan syndrome is said to affect ethnic groups and males/females equally. However, in our analysis of published case studies, sex was reported in 920 patients; we found that 59% of them were male and 41% were female. Our analysis was not comprehensive nor was it designed to make an accurate estimate of sex ratios in NS. Consequently, we do not know how accurate this figure is.
Growth curves for Noonan syndrome are available on our Brazil page. The curves were made using data from Brazilian NS patients.
The clinical features of NS are variable, and there is no single set of defining features for this syndrome. In addition, features vary in severity and some, such as facial appearance, change with age. These facts complicate diagnosis. We analyzed case studies reporting on just over 1,000 patients and found that no single clinical feature occurred in more than 90% of patients, and that few occured in 75% or more patients. This finding may have been due in part to the changing facial appearance in NS as patients age (2), and incomplete reporting of clinical features in different studies. However, the features listed below occured in 75-87% of people with NS in our analysis.
- Cardiac abnormalities
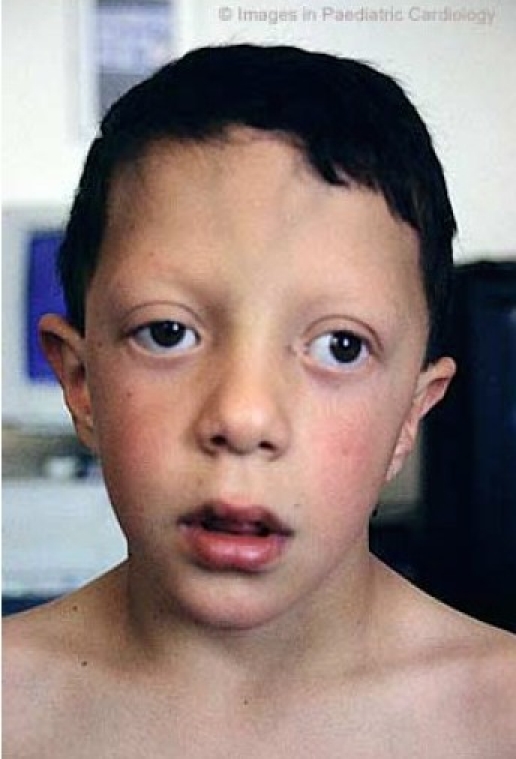
- Ears that sit low on the head
- Ears rotated and pointing toward back of head
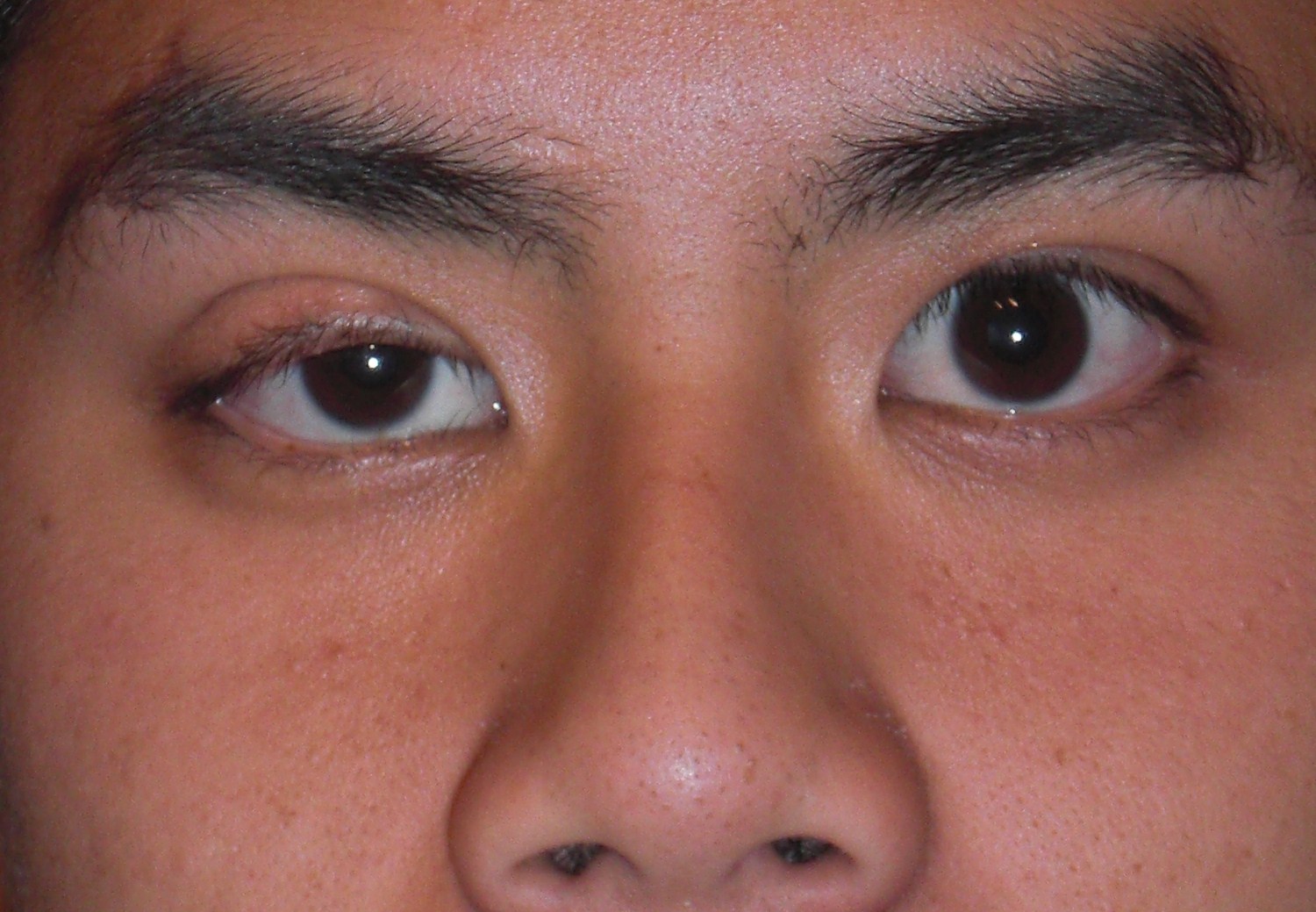
- Downslanting palpebral fissures (eyes slant downward)
Very common features of Noonan syndrome
Like many genetic conditions, NS affects a variety of body systems. In particular, cardiac problems are of note in NS, as are skeletal abnormalities (especially the chest and hands), and problems with the eyes and vision. Combinations of specific problems that occur in a single person can aid diagnosis. The following are common problems in NS:
- Sunken chest and pigeon chest occurring together (pectus excavatum and pectus carinatum)
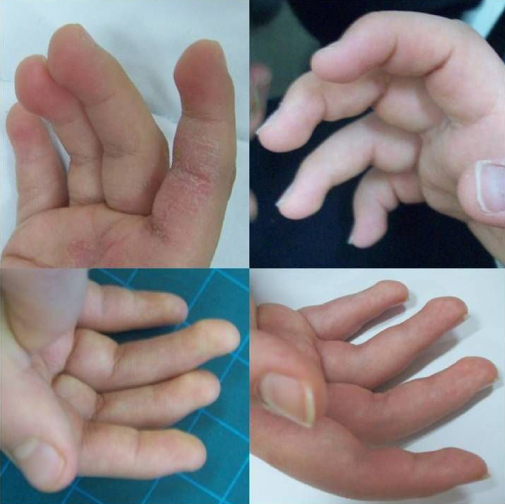
- Fetal finger pads (lumps on fingertips; see photos below)
- Atrial or ventricular septal defects
- Wide chest/widely spaced nipples
- Undescended testicles in boys
- Hypertrophic cardiomyopathy
- Pulmonary valve stenosis
- Other cardiac defects
- Intellectual disability
- Dental abnormalities
- High-arched palate
- Loose/lax joints
- Vision problems
- Short stature
Common clinical features of Noonan Syndrome
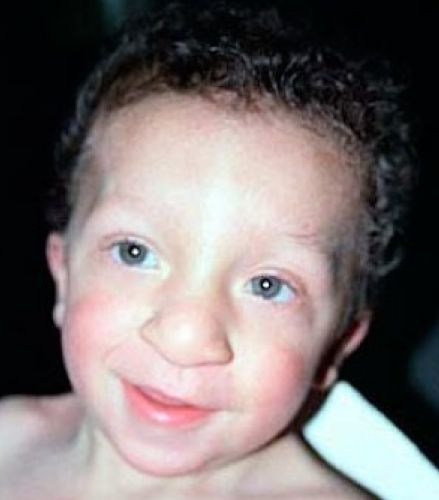
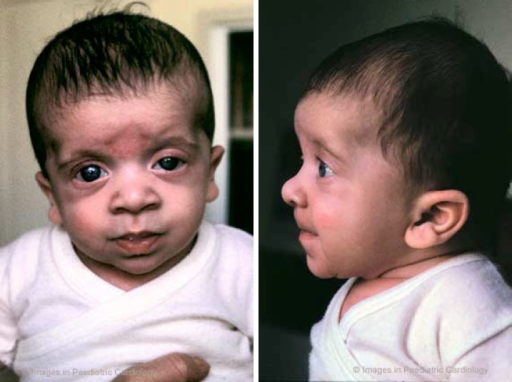
Many people with NS have a characteristic facial appearance (see photos on this page). As noted, these characteristics typically change as a patient ages. For a set of examples, the pictures of a boy with NS at two different ages. The table below has information about facial features of NS and is adapted from references 2 and 3:
Facial features of NS by age |
|
Infancy |
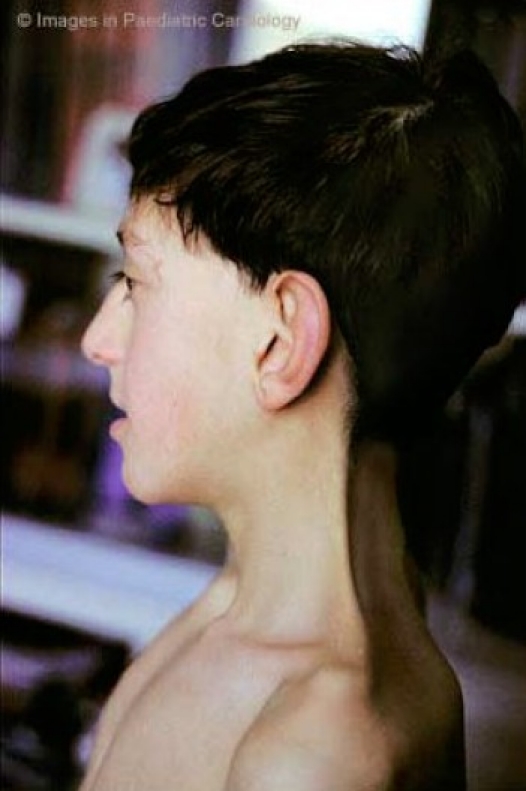
Highly arched palate Small jaw Relatively large head Tall forehead Short neck Excess skin on back of neck Wideset eyes Downslanting palpebral fissures Short, broad nose with flat tip Depressed nasal root Low-set ears that point toward the back Thick ear helices |
Childhood |
Face lengthens Face becomes triangular Upper face broad Chin: narrow and pointed |
Adolesence |
Nose has thin, high bridge High hairline in front Neck becomes longer Neck webbing is more apparent |
Older adults |
Nasolabial folds prominent Thin skin Transparent skin |
Most people with Noonan syndrome have normal intelligence. As an example, intelligence was reported in 500 patients in in our analysis, and 189 had an intellectual disability (38%).
Some people with NS have a low IQ, while others may have a normal IQ and learning disabilites. For example, vision/heasupportring problems and fine motor delays can interfere with learning. One study of IQ in NS found an average IQ of 84 among 48 children with a range of <50 (2 people) to 120-129 (1 person; 4). The same study found that the children tested tended to have difficulties with explaining the meaning of a word or elaborating on similarities between things. Many also had trouble on subtests that measured a person's ability to pay attention.
The prevalence of NS is usually cited as being roughly 1 in 1,000 to 2,500 (5). However, this figure is old (6) and was not calculated using a population study. If the estimate is correct, there are 124,000 to 310,000 people with NS in the United States.
Diagnosis and Testing
Diagnostic criteria for NS have been established (7). The major criteria include typical facial features, the cardiac abnormalities specified in the list above, short stature (<3rd percentile; infants may be long at birth but may fall off curves quickly), pectus carinatum/excavatum, a first degree relative with NS, and all of: intellectual disability, cryptorchidism in males, and lymphatic vessel dysplasia. The minor criteria include suggestive facial features, any cardiac abnormality not noted above, height <10th percentile, a broad chest, a first degree relative with features suggestive of NS, and one of: intellectual disability, cryptorchidism in males, or lymphatic vessel dysplasia.
Noonan syndrome is an autosomal dominant disorder. This term means that only one copy of a mutated gene is needed to cause the disorder. Most patients have an affected parent, although sporadic cases also occur. In a sporadic case, the mutation occurs spontaneously, typically with no known reason. It is caused by mutations in a number of different genes: it is most commonly caused by mutations in PTPN11. Mutations in SOS1 are the next most common cause of NS. Other genes associated with NS are BRAF, KRAS MAP2K1, NRAS, and RAF1. The link at the right provides information about labs that test for NS.
Differential Diagnosis
NS is a relatively common condition. As noted, it is variable and can be hard to definitively diagnose on the basis of clinical features alone. Molecular methods can help, but there is also overlap between mutated genes and different diseases. A number of conditions share similarities with NS.
Noonan Syndrome with multiple lentigines (NSML). This syndrome was formerly called LEOPARD syndrome, which referred to multiple lentigines (spot-like lesions) on the skin of patients. The letters in the acronym stand for Lentigines, Electrocardiographic conduction abnormalities, Ocular hypertelorism, Pulmonic stenosis, Abnormal genitalia, Retardation of growth, and sensorineural Deafness (8). It was renamed because the name was offensive and because it is caused by mutations in the same genes that cause Noonan syndrome.
NS and NSML share many features, including short stature, similar cardiac and chest abnormalities, and a number of similar facial features (ptosis and wideset eyes, for example). The presence of numerous dermal lentigines (many thousands) is an important feature distinguishing NSML from NS, although the lentigines are not present in all patients. NS and NSML are allelic syndromes, meaning that they can be caused by mutations in the same genes (PTPN11, RAF1, BRAF).
Cardiofaciocutaneous (CFC) syndrome. CFC syndrome is also a RASopathy. Like Noonan syndrome, CFC causes cardiac abnormalities and lymphedema (tissue swelling caused by problems in the lymphatic system, including blocked lymph channels). There is also overlap in the genes that cause NS and CFC syndrome. However, intellectual disability is generally more severe in CFC syndrome, as are problems in the gastrointestinal tract. People with CFC syndrome may also have anatomical abnormalities that do not generally occur in NS.
Costello syndrome (CS). Costello syndrome is another RASopathy. Many patients have cardiac abnormalities, including hypertrophic cardiomyopathy. Other features shared between the two conditions include short stature, intellectual disability, lax joints, and head/neck features such as a highly arched palate, a short neck, a large forehead, and low-set ears. Features that can help distinguish them include normal intelligence (>99% of Costello Syndrome patients are intellectually disabled), and sagging or loose skin (>95% of CS patients have this problem). Costello syndrome is caused by mutations in the gene HRAS, and molecular methods can distinguish it from Noonan syndrome.
Neurofibromatosis type 1 (NF1). Although NF1 is a RASopathy like NS, the phenotypes of these two conditions are not generally as close as is the case with other conditions. NF1 patients tend to have multiple benign tumors that do not occur in NS. NF1 patients are also at high risk for CNS gliomas and nerve tumors. NF1 is caused by mutations in the gene NF1.
However, a minority of NF1 patients have what is called a Noonan syndrome phenotype (9). These individuals have a facial appearance that is similar to NS and may also have neck webbing and pulmonary stenosis. Their first degree relatives may or may not have the same appearance and problems. They may even have mutations in the gene PTPN11, though most have mutations in NF1.
References
- 1. Jongmans MC et al. (2011) Cancer risk in patients with Noonan syndrome carrying a PTPN11 mutation. Eur J Hum Genet 19(8):870-874. Full text on PubMed.
- 2. Allanson JE et al. (2010) Noonan syndrome: The changing phenotype. Am J Med Genet 21(3):507-514. Abstract on Pubmed.
- 3. Allanson JE et al. (2010) The face of Noonan syndrome: Does phenotype predict genotype. Am J Med Genet A 152A(8):1960-1966. Full text on PubMed.
- 4. Lee DA et al. (2005) Psychological profile of children with Noonan syndrome. Dev Med Child Neurol 47(1):35-38. Abstract on Pubmed.
- 5. Tartaglia M et al. (2010) Noonan syndrome: clinical aspects and molecular pathogenesis. Mol Syndromol 1(1):2-26. Full text on PubMed.
- 6. Nora JJ et al. (1974) The Ullrich-Noonan Syndrome (Turner Phenotype). Am J Dis Child 127(1):48-55. Abstract on Pubmed.
- 7. Bhambhani V & Muenke M (2014) Noonan syndrome. Am Fam Physician 89(1):37-43. Full text on PubMed.
- 8. Gorlin RJ et al. (1969) Multiple lentigines syndrome: complex comprising multiple lentigines, electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of genitalia, retardation of growth, sensorineural deafness, and autosomal dominant hereditary pattern. Am J Dis Child 117(6):652-662. Citation on Pubmed.
- 9. Friedman JM (1998) Neurofibromatosis 1. Updated September 4, 2014. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 10. Photo found in the Wikimedia Commons.
- 11. Digilio MC & Micale L (2001). Clinical manifestations of Noonan syndrome. Images Paediatr Cardiol 3(2):19-30. Full text on PubMed.
- 12. Ko JM et al. (2010) Phenotypic and cytogenetic delineation of six Korean children with Kabuki syndrome. J Genet Med 7(1):37-44. Full text.