Osteogenesis imperfecta (type 1)
Osteogenesis imperfecta, or OI, is a family of disorders whose most obvious feature is fragile bones that are prone to breaking. There are many types of OI, but brittle bones and fractures are common to all of them. This is why OI is sometimes called brittle bone disease. The severity of OI ranges be mild to severe, with the mild form being the most common.
OI is formally classified into groups numbered from 1 to 5 (1, 2), with type 5 added in the early 2000s (3). Many groups also have classifications with additional forms. Type 1 is mildest, and life expectancy is roughly the same as for the general population. A person with type 1 OI may suffer only a few to dozens of broken bones in a lifetime. Some people with type 1, especially those with few breaks, aren't diagnosed until adulthood.
Type 2 is the most severe form of OI, and patients tend to die before birth or very soon afterwards. Type 3 can also be a severe form, and many patients suffer broken bones before birth. These individuals may have dozens to hundreds of fractures during their lives, with some having an average lifespan, and others dying in early adulthood or sooner. Types 4 and 5 are intermediate. A puzzling thing about OI is that severity varies widely, even among patients with the same type --- and even among members of the same family. The reasons for these differences are not well understood.
Many newer studies have argued that there are additional subtypes of OI, such as type 6, which is caused by a gene not associated with other diseases or other forms of OI (4, 5). However, a very recent paper left the classification at 5 subtypes, noting that overlap among the candidates for new subtypes (1). The classification scheme for OI doesn't seem to have been finalized, however. The Ostegenesis Imperfecta Society has a good description of the types of OI, including some beyond type 5.
Overall, OI is very rare, occurring in 1 birth in 13,500 to 15,000 (reviewed in 1). This estimate doesn't include milder forms that aren't typically diagnosed until later in life.
The earliest known cases of OI date to roughly 2,000 to 3,000 years ago. One was a teenaged girl who lived in the Great Lakes region of what is now Canada (6). She lived roughly 3,000 years ago and died at age ~16. She's thought to have had type 4 OI. She was short for her age, with an estimated height of 120 cm (3 feet, 10 inches). An analysis of her bones indicated that she wouldn't have been able to walk, and that use of her arms was also impaired.
Another patient from the same time has been found in Egypt (7, 8). This person was an infant who was mummified and buried in Beni Hassan, an ancient cemetary. His or her leg bones were severely bowed (7). Another infant, thought to have died at birth at ~38 weeks gestation, was also found in Egypt, at Dakhleh Oasis. This child is believed to have died 2,000 years ago and had a severe form of OI. As with the the other infant, this child's legs were also bowed. Numerous fractures were also seen (9).
Clinical information
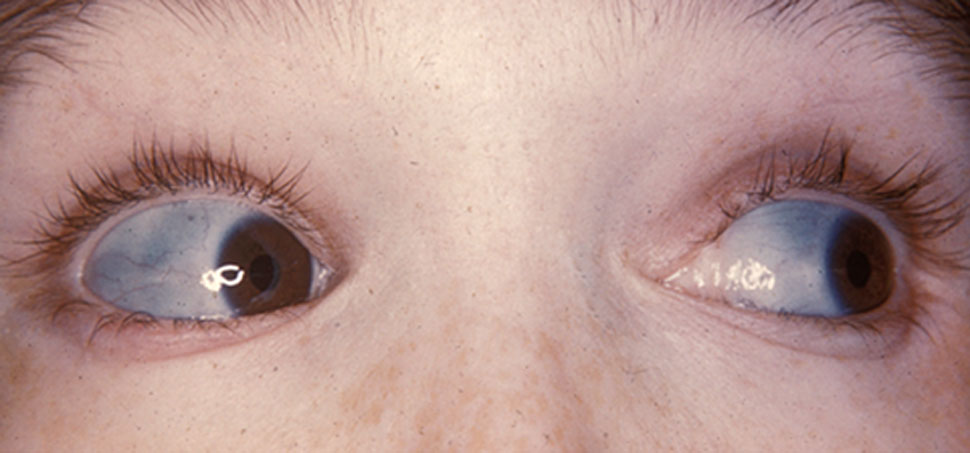
Type OI 1 mostly causes problems the musculoskeletal system, but other systems are also affected. Its most consistent features are fragile bones that can break after relatively minor trauma, such as during normal play. Patients may also have blue sclerae (the whites of the eyes are blue-ish or grey-ish; see photo above). Blue sclerae may occur up to age 18 months in children without OI. Both of these problems occurred in 95% or more of patients in our literature survey (information known for ~160 patients). Broken bones become less common in adulthood. Dental abnormalities are also common in type 1 OI, occurring in 81% of ~100 patients for whom information was available.
The most common signs and symptoms of OI type 1 are as follows:
- Fragile bones that break easily or more easily than would be expected in the general population
- Osteopenia or osteoporosis (low or very low bone density) in a person under age 50
- Blue sclerae (the whites of the eyes are blue-ish; see photo at top of page)
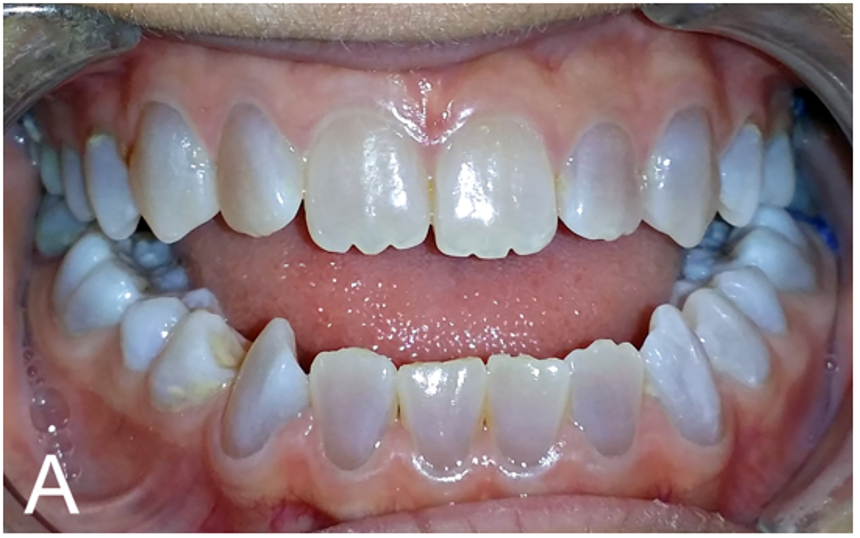
- Dental abnormalities, such as discoloration or translucency
- Wormian bones (extra bone pieces in a suture in the skull)
- Bowing of the long bones (arm and leg bones; see images)
- Compression fractures of the vertebrae
- Delays in motor development
- Short or very short stature
- Joint laxity/hypermobility
- Hearing loss
- Scoliosis
- Flat feet
Clinical features of OI type 1
OI type 1 does not appear to affect cognitive ability/IQ. A small minority of patients have heart disease.
DGI is a common problem in OI. It affects dentin, which is one of the major components of teeth. The result is that a patient's teeth are weaker than normal, making them prone to breaking, wearing down, and falling out. They're also discolored and translucent (light passes through them). The photo on this page shows an OI 1 patient with DGI.
In our literature survey, DGI was found in 40% of 100 patients for whom information was available.
Cause, Diagnosis, and Testing
Type 1 OI is caused by mutations in COL1A1 or COL1A2. These genes make type 1 collagen, which is an important building block in bones. Basically, collagen holds the body together and gives it strength. People with type 1 OI make normal collagen, but not enough of it. This is why their bones are brittle. Collagen is also an important part of other tissues, like tendons and ligaments, which is why some OI patients have lax/loose joints.
OI type 1 is an autosomal dominant disorder. The term autosomal dominant means that a disease can be passed from one affected parent to a child. Thus, many people with type 1 OI have a parent who also has it. When we did our literature review, we found that 75% of 70 people with OI type 1 had a parent who also had it. In the other cases, the disease occurred sporadically in a family without a history of OI. In these cases, the mutation occurred spontaneously in an egg or sperm for reasons that are not known.
Unfortunately, there are no formal criteria for diagnosing OI type 1. A GeneReview article notes that common features of OI include fractures, short stature and bone deformity, blue sclerae, DGI, hearing loss after puberty, lax joints and ligments, and a family history of OI (10). Note that fragile bones are common to all types of OI, and they can't distinguish subtypes.
Certain lab tests can help confirm a diagnosis of OI. For example, X-rays may show bones that are slightly bowed when this problem is not visible to the eye. Bone are typically thin-looking. In addition, tests for collagen can help. As noted, people with type 1 OI tend to have low levels of normal collagen. In contrast, people with other types of OI also have low levels of collagen, but the collagen they make is also abnormal. This lack of even some normal collagen is presumably what makes other forms of OI more severe than type 1.
A diagnosis suspected by clinical features can be confirmed by finding a mutation in COL1A1 or COL1A2. For a list of labs that perform testing, see the links at right.
Treatments and Management
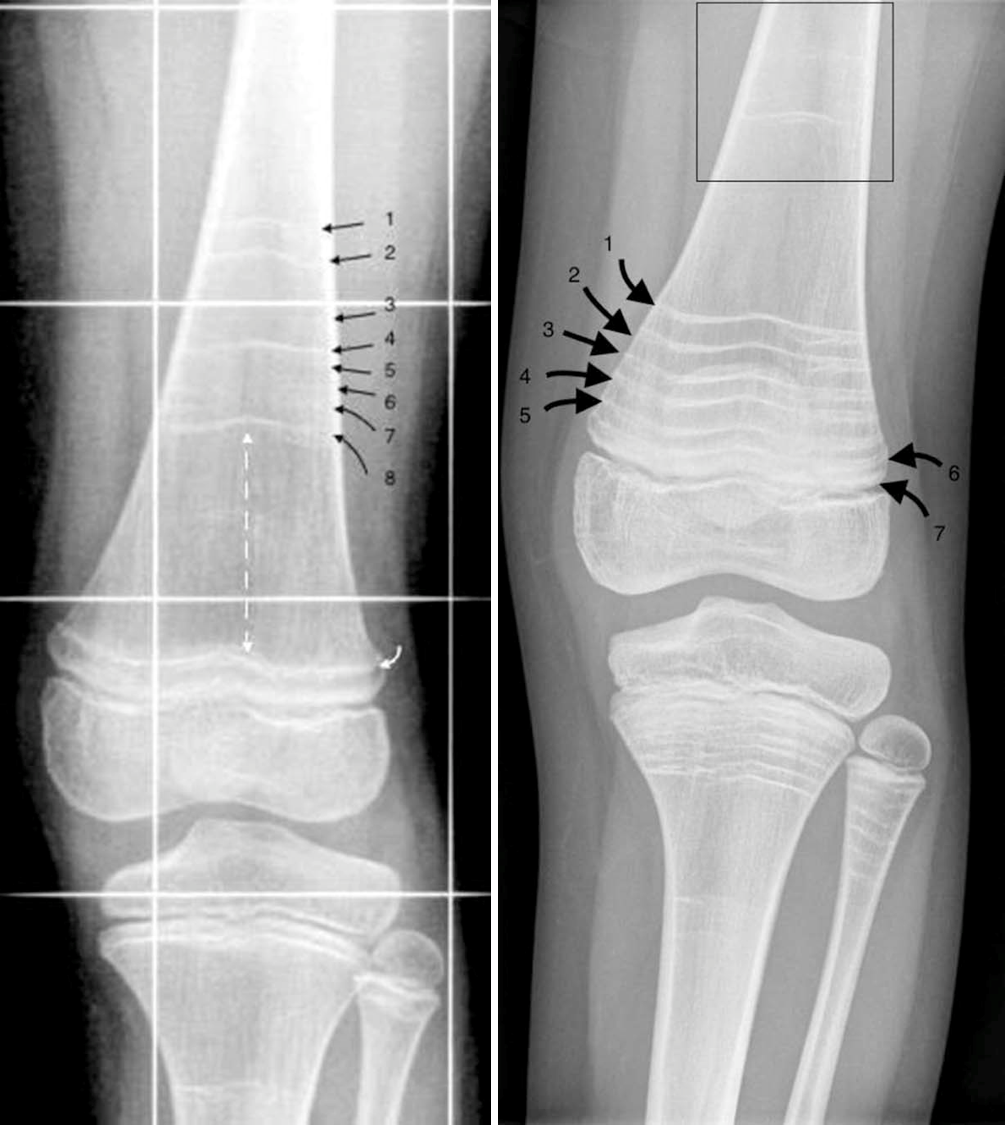
There are no cures for any form of OI. Doctors focus on managing OI, such as by minimizing fractures, minimizing disabilities, and helping a person live as independently as possible (10). Physical therapy is an important tool for maintaining mobility and after injuries. Medicines are also important. For example, bisphosphonates including pamidronate (11) are commonly used in OI. Bisphosphonates are drugs used to prevent loss of bone density and reduce the number of broken bones. For example, pamidronate is typically infused over the course of several years.
Surgeries are also commonly used to help people with OI. For example, surgery may be used to correct curved bones or to stabilize the spine. Rods can be inserted to help support bones. Surgery can also be used to help with hearing loss. For a full discussion on managing all types of OI, see reference 10. Also, the Ostegenesis Imperfecta Society has a good summary of treatments for OI. Ideally, people with OI should have access to a diverse team of specialists who can help them.
Differential Diagnosis
OI type 4. This type of OI is also called the moderate form. Unfortunately, this term can be misleading because severity varies from being relatively mild (only a few fractures over the course of a life) to severe (fractures present at birth). Thus, in its milder presentations, fracture frequency in the milder form of type 4 overlaps with type 1. People with OI types 1 and 4 may also have similar facial features (a triangular face and a prominent forehead, for example) and abnormal spinal curvature. Testing for genetic mutations may distinguish the two conditions, because type 4 OI is associated with a number of genes apart from COL1A1 and COL1A2. They include CRTAP, PPIB, FKBP10, SERPINF1, and WNT1.
OI type 5. Type 5 OI is a moderate form of OI. Similarities with type 1 include short or very short stature, scoliosis and other problems involving curvature of the spine, bowing of the long bones, and loose or hyperexensible joints. Blue sclerae are relatively rare in OI type 5, but relatively common in type 1. A very common feature of type 5 OI is calcification (hardening) of a tissue called the interosseous membrane, or IM. The IM connects the two bones in the forearm and keeps them stable (see images on our type 5 OI page). When the IM hardens, it loses flexibility, and a person begins to have trouble moving his lower arm. The most common problem is difficulty rotating the palms up and down when the arm is bent at the elbow. Another common problem in type 5 OI is calcified masses of tissue around the sites of fractures (hyperplastic calluses). Genetic testing can give a definitive answer: OI type 5 is only caused by mutations in IFITM5, whereas OI type 1 is cause by mutations in COL1A1 and COL1A2.
Non-accidental injury (child abuse). When a child breaks a bone without any obvious cause, it's natural to suspect abuse. This may especially be the case if a child has a mild form of OI. Obviously, however, confusing OI and abuse would likely have disastrous results for the misdiagnosed child. X-rays and a careful family history can help distinguish the two. A recent review on radiographic features of OI contrasts findings that are more typical with OI and those that are more typical of child abuse (12). In summary, the review notes that the main features X-ray features of OI include osteopenia, fractures, and bone deformities. Note that osteopenia can be difficult to see on an X-ray and DEXA scanning is currently the best method for diagnosing it, provided that people who interpret results are knowledgeable about differences related to age, sex, stage of puberty, and skeletal maturation (12). In patients with osteopenia, it is also important to exclude other cuases, such as diabetes, vitamin D deficiency, and other conditions.
Reference 12 has a table listing differences between radiologic findings in child abuse and OI. It is highly recommended, along with consultation by a medical doctor who is knowledgeable about OI. Differences include fractures to the posterior ribs and complex skull fractures (common in child abuse victims but not in OI), deformed long bones and and osteopenia (common in OI but not in child abuse victims), and a set of other findings too numerous to list here. Again, consultation with an expert physician is essential.
Rickets. Rickets is a condition typically caused by vitamin D deficiency. It can also be caused by calcium deficiency. Its clinical features include bowed legs, short or very short stature, bone pain, trouble sleeping, and a large forehead. Patients may also suffer broken bones and scoliosis. Rickets is most common nutritional problems are prevalent and where children lack exposure to the sun. Low serum levels of vitamin D or calcium can distinguish it from OI. Reference 13 has a good overview of this disorder.
Dentinogenesis imperfecta/DGI, types 2 and 3. OI isn't the only disorder that causes DGI. It is also caused by mutations in the gene DSPP, which makes two proteins that are essential for normal tooth development. These forms of DGI are called types 2 and 3. They do not appear to increase the risk of bone fractures beyond the teeth. For a review, see NORD's review of DGI type 3.
For a longer list of conditions in the differential diagnosis of OI, see this document on the OI Foundation's website.
References
- 1. Tournis S & Dede AD (2017) Osteogenesis imperfecta - a clinical update. Metabolism 80:27-37. Abstract on PubMed.
- 2. Sillence DO et al. (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16(2):101-116. Full text on PubMed.
- 3. Glorieux FH et al. (2000) Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res 15(9):1650-1658. Full text from publisher.
- 4. Glorieux FH et al. (2002) Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res 17(1):30-38. Full text from publisher.
- 5. Homan EP et al. (2011) Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res 26(12):2798-2803. Full text on PubMed.
- 6. Vairamuthu T & Pfeiffer S (2018) A juvenile with compromised osteogenesis provides insights into past hunter-gatherer lives. Int J Paleopathol. 20:1-9. Full text from publisher via PubMed.
- 7. Gray PHK (1970) A case of osteogenesis imperfecta, associated with dentinogenesis imperfecta, dating from antiquity. Clin Radiol 21(1):106-108. Abstract on PubMed.
- 8. Lowenstein E (2009) Osteogenesis imperfecta in a 3,000-year-old mummy. Childs Nerv Sys 25(5):515-516. Abstract on PubMed.
- 9. Cope DJ & Dupras TL (2011) Osteogenesis imperfecta in the archeological record: an example from the Dakhleh Oasis, Egypt. Int J Paleopathol 1(3-4):188-189. Abstract on PubMed.
- 10. Steiner RD et al. (2005) COL1A1/2-related osteogenesis imperfecta. Updated 14 February 2013. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 11. Blouin S et al. (2017) Hypermineralization and high osteocyte lacunar density in osteogenesis imperfecta type V bone indicate exuberant primary bone formation. J Bone Miner Res 32(9):1884-1892. Full text on PubMed.
- 12. Renaud A et al. (2013) Radiographic features of osteogenesis imperfecta. Insights Imaging 4(4):417-429. Full text on PubMed.
- 13. Creo AL et al. (2016) Nutritional rickets around the world: an update. Paediatr Int Child Health 37(2):84-98. Abstract on PubMed.
- 14. Andersson K et al. (2017) Mutations in COL1A1 and COL1A2 and dental aberrations in children and adolescents with osteogenesis imperfecta - a retrospective cohort study. PLoS One 12(5):e0176466. doi: 10.1371/journal.pone.0176466 Full text on PubMed.
- 15. Loizidou A et al. (2017) Pamidronate "zebra lines": a treatment timeline. Radiol Case Rep 12(4):850-85.3. Full text on PubMed.
- 16. Fred HL et al (2008) Images of Memorable Cases: Cases 40, 41 & 42 OpenStax CNX. Dec 3, 2008 http://cnx.org/contents/fe89fbf7-c641-4ad8-8871-80017adfd2cf@3. Photograph on cnx.org.