Nijmegen Breakage Syndrome (NBS)
Nijmegen Breakage Syndrome, or NBS, is an inherited disease that causes abnormalities of growth, development, and immune system. People with NBS also have a very high cancer risk.
NBS has been classified as a member of different groups of diseases. One of them is the DNA repair disorders. All of these disorders involve problems in fixing DNA that has been damaged by a variety of agents including ultraviolet light from the sun and flourescent lights, ionizing radiation from the cosmos, X-ray equipment and other medical devices, chemicals, normal internal processes in cells, and other substances or processes. Damage to DNA is a constant, and living things have evolved many systems for fixing it. For example, there are proteins that recognize that DNA is damaged, enzymes that remove the damaged sections, other enzymes that unzip a strand of DNA so that other molecules can get enter and make repairs, and enzymes that insert new, undamaged DNA bases. This list is not exhaustive. When a person has a defect in one of these repair systems, disease results. Overall, DNA repair disorders greatly increase the risk of cancer.
NBS is also a primary immunodeficiency. This term means that there are problems with the immune system that are inherited. This is in contrast to immunodeficiencies like AIDS, which are caused by infection, a medication, or some other external factor. There are many primary immunodeficiencies. One of them, ataxia-telangiectasia, (A-T) is also a DNA repair disorder. People with these diseases have trouble fighting infections. For example, NBS patients are susceptible to bronchitis and pneumonia.
NBS has been known by a variety of names, inclucing A-T variant 1, A-T variant 2, Berlin breakage syndrome, Czech breakage syndrome, and Seemanova syndrome (3).
NBS affects males and females equally. Although it appears to occur around the world, it is more common among people of Eastern or Central European origin, such as those from the Czech Republic, Poland, Russia, and Ukraine (1, 2). A large majority of published case histories reviewed by us fit this description (83% of ~150 patients were of Slavic origin). Roughly 90% of Slavic patients share the same mutation (1). Other mutations occur in other groups of people (3). NBS is not evenly distributed globally (2), but it is rare or very rare in all populations. There are no precise estimates for its prevalence or incidence (1, 3), but one review has estimated incidence at 1 birth in 100,000 (3).
Clinical information
A key feature of NBS is microcephaly, or a very small head. This problem is usually evident at birth, thought it may appear in the first year of life in some patients. It occurs in nearly all patients with NBS, and is progressive, which means that it gets more pronounced with time. This is because the rate of head growth slows down relative to body growth. As a result, the percentile value of a patient's head size drops in comparison to his body size and to other children his age. Thus, if a child's head circumference at birth is on the 8th percentile, it might drop to the 1st percentile by age 1, and continue to fall after that. While children with NBS tend to be small, in general, growth retardation of the head is more severe than growth retardation of the body.
Surprisingly, cognitive development/IQ is normal in a sizable minority of NBS patients, in spite of severe microcephaly. In addition, early development of motor skills is often normal. These facts contrast to what would be expected from a child with very poor head growth. Head growth is used as a proxy for brain growth, and poor brain growth tends to indicate poor brain development. Our literature survey found that intelligence was normal in ~1/3 of 100 patients, all of whom had microcephaly. Similarly, 85% of children met all motor milestones from birth to age one in spite of microcephaly. In fact, the combination of severe/progressive microcephaly with normal early childhood development is suggestive of NBS.
Another hallmark of NBS is immunodeficiency of both the cellular and humoral responses (1). In practical terms, this phrase means that NBS patients tend to get frequent infections. Diseases that are common in NBS include ear infections (otitis media), colds and flu-like illnesses, and lower respiratory infections. Pneumonia is a special concern, as it has caused death in some NBS patients (4).
Common signs and symptoms of NBS are listed below (1, 4); as with any medical condition, individual patients may lack some of the clinical features noted.
- Microcephaly (very small head; growth falters as time passes)
- Immunodeficiency (pneumonia can develop at a young age)
- Frequent infections (respiratory, gastrointestinal, urinary)
- Malignancies/cancers (especially lymphomas)
- Premature ovarian insufficiency in females
- Delayed puberty in both boys and girls
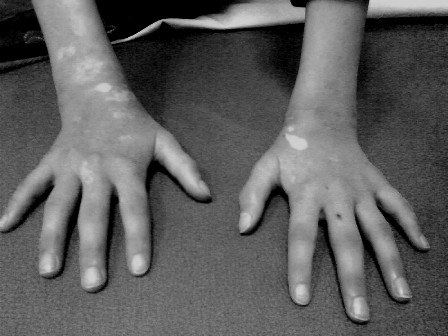
- Areas of abnormal skin pigmentation
- Syndactyly (fingers or toes fused)
- Delayed speech development
- Infertility in both sexes
- Low birthweight
- Short stature
- Sparse hair
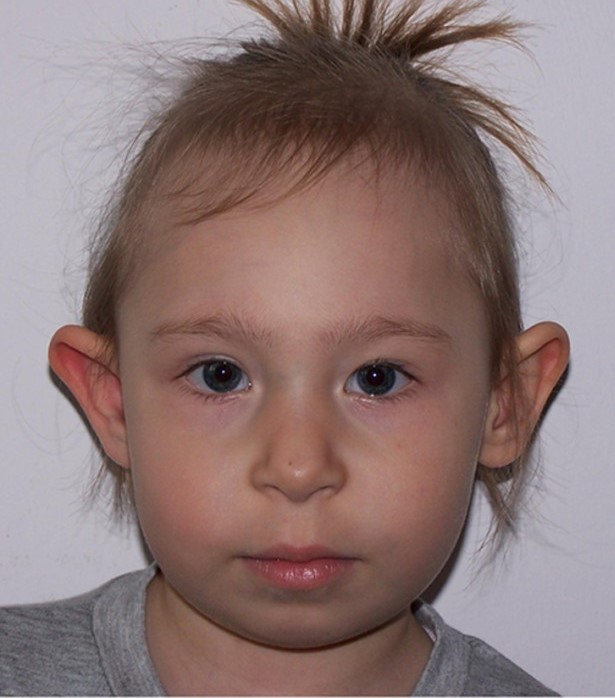
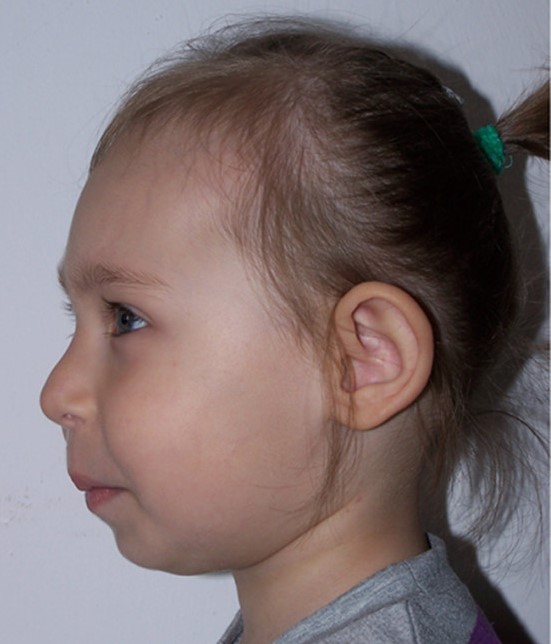
Clinical features of NBS
- Receding forehead (slopes backward)
- Receding/underdeveloped jaw
- Prominent rounded midface
- Large or prominent ears
- Upward slanting eyes
- Wideset eyes
- Long nose
Facial features of NBS
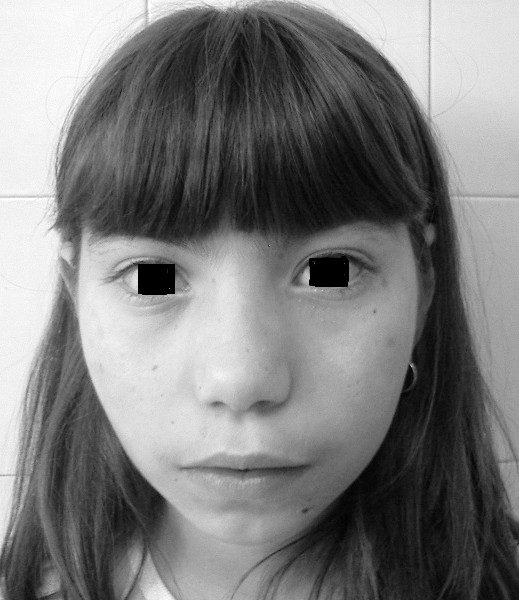
In addition to the clinical features listed above, all NBS patients have similar facial features. The most common
ones are listed below. See the photos on this page for examples.
These features occurred in a majority of patients in our case history review. The combination of a receding forehead and a receding chin results in a side profile which appears rounded (see photo at right on this page). Facial features in NBS may become more obvious with time.
Cause
NBS is an autosomal recessive disorder caused by mutations in the gene NBN. The term autosomal recessive means that the disorder is passed on when both parents contribute a copy of the mutated gene to their child. It also means that siblings of child with NBS have a 25% probability of also having the disorder, and 50% chance of being carriers. In most cases, being a carrier does not cause health problems. However, NBS carriers have elevated risk of developing cancer (see below; 3, 5, 6). Thus, people related to an NBS patient should be aware that they may be carriers, and if they are, should be checked frequently for signs of cancer.
The NBN gene makes a protein that has an important role in repairing double strand breaks in DNA, which are breaks to both strands of the DNA molecule (1). Ionizing radiation including X-rays causes these breaks; an NBN patient's impaired ability to repair these breaks can create serious problems if radiation therapy is used to treat cancer (see below).
Diagnosis and Testing
Diagnosis of NBS relies on a combination of clinicial features and laboratory testing. Clinical features that are suggestive of NBS are as follows. The following information has been adapted from reference 1.
-
Leading symptoms
- Microcephaly, which is usually present at birth
- Frequent respiratory infections
- Malignancies, especially of the hematologic type (e.g. leukemia, lymphoma); solid tumors may also occur
- A characteristic facial appearance (jaw, forehead, and midface in list above; see photo)
- Mild retardation of body growth
- Premature ovarian sufficiency in females
- Good development of motor skills and overall absence of neurological symptoms
- Cognitive development is better in early childhood but declines gradually (intelligence may be normal or borderline initially)
Additional criteria
Other factors that are supportive of a diagnosis of NBS include siblings with microcephaly or hydrocephaly (fluid buildup in the brain), early death due to cancer or infections, or other family members with cancers.
Finally, certain tests can support a diagnosis of NBS. They include laboratory tests that indicate immunodeficiency, chromosome instability, and/or sensitivity to ionizing radiation (1, 3).
Gene sequencing can confirm a suspected diagnosis of NBS. In patients with Slavic ancestry, testing for an NBN mutation called c.657_661del5 (also called 657del5)is the recommended route (1). This mutation is very common in this NBS patients of Slavic ancestry. If this methodology is not available, cytogenetic analysis/karyotype analysis is recommended as the next best choice. Comparative genomic hybridization is noted as being inappropriate (1). For additional information about labs which conduct such testing, please see the link to the right.
Cancer and NBS
As noted, cancer is common in NBS. It was reported in 41% of 124 patients in our literature survey, most often lymphoma (occurred in 27% of all patients) and leukemia (7% of all patients). Other surveys of NBS patients have found a similar cancer rate (1, 3). NBS patients should be monitored closely for signs of malignancy.
Radiation therapy is a common treatment for many cancers. However, due to extreme radiosensitivity in the NBS population, it is likely to cause severe adverse reactions, and may even be be fatal (1, 7, 8). Thus, the consensus among experts in this disease is that radiation should be avoided in NBS. In addition, anti-cancer drugs that mimic the effects of radiation therapy should also be avoided (1, 3).
A study that followed children with NBS over time found that 40% of patients experienced a sudden rise in IgM levels. Roughly 63% of this group developed lymphomas after the dramatic change in IgM (9). Another study examined the possible role of Epstein-Barr virus infection in oncogenesis in NBS patients (10). While neither link has been proven, clinicians who are caring for NBS patients might wish to review references 9 and 10 and to increase their awareness of potential problems whose effects may be reduced through careful monitoring.
Research studies have found elevated cancer risk in carriers with 657del5 mutations (5, 11-15). Carrier risk is not as high as that of NBS patients, but it is elevated compared with non-carriers. As a result, carriers should be identified, counseled about their risk, and offered frequent monitoring for the development of cancer.
Differential Diagnosis
A number of other conditions are similar to NBS. A few of them are listed below. Others are described in tables in the Gene Reviews article for NBS (3) and in the recent review of NBS (1).
Fanconi Anemia (FA). FA is a common misdiagnosis among NBS patients. Key features of both conditions include microcephaly (with or without normal intelligence), susceptibility to infections, small body size, abnormalities of the kidneys and other internal organs, and cancer susceptibility. In contrast to NBS patients, low platelet counts are near-universal in FA, though they are not necesarily present at very young ages. Other hematologic abnormalities include anemia and low white cell counts (overall or neutropenia). Pancytopenia is common in FA (low platelets, anemia, and low white cell counts)m but not in NBS. Bone marrow failure is also common in FA. In addition, abnormalities of the thumbs are suggestive of FA (extra thumbs, malformed thumbs, or bifid thumbs). Thumb abnormalities have been reported in NBS, but only very rarely (15, 16). Thus, while a thumb malformation cannot rule out NBS, it is nonetheless more suggestive of FA.
Ataxia-Telangiectasia (A-T). A-T and NBS are similar in many ways in that both feature immunodeficiency, high risk for cancer development, and chromosomal instability occur. However, NBS patients seldom have progressive neurological features that define A-T, such as ataxia, gaze abnormalities, stooping posture, and loss of facial expression. Telangiectasias have been observed NBS patients, and while they are relatively rare, their presence should not be used to rule out NBS. The key differences between the two conditions are in the progressive neurological problems that essentially define A-T, yet are essentially absent from NBS. A-T is caused by mutations in the gene ATM, and can therefore be distinguished from NBS by gene sequencing.
A-T Like Disorder 1 (ATLD-1, MRE11A defect). ATLD-1, like NBS, features microcephaly and unusual sensitivity to ionizing radiation. Ionizing radiation sensitivity is also a problem in A-T. Unlike NBS, ataxia is important feature of ATLD-1. This trait makes it resemble A-T more than NBS. Ataxia onset in ATLD-1 is slow but progressive (gets worse with time). Telangiectasias and immunodefiencies do not appear occur in this condition (1). In contrast with NBS, ATLD-1 patients have been reported to lack cancers and immunodeficiencies (17-19). However, very few patients have been reported in the literature, and patients with these problems may described in time.
LIG4 syndrome. People with this syndrome have microcephaly and growth retardation. Like NBS patients, head growth is more seriously affected than body growth. Facial features are similar between the two conditions, and LIG4 patients also have immunodeficiency, sensitivity to ionizing radiation, and increased risk for the same types of malignancies that affect NBS patients. LIG4 patients are also prone to pancytopenia (low platelets, anemia, and low white cell counts). Both NBS and LIG4 syndrome involve chromosomal instability, but the specific problems are different. Gene sequencing can also distinguish the two conditions.
Nijmegen breakage syndrome-like disorder (RAD50 deficiency). RAD50 Deficiency appears to be extremely rare, with only a single case reported in the literature (20). The patient was initially diagnosed with NBS, given her clinical features of microcephaly, characteristic facial features, skin pigmentation abnormalities and short stature. However, she had no immune disturbances and did not develop malignancies. She developed normally during puberty, and was also reported to have a minor and nonprogressive ataxia, unlike NBS patients (20). It is unknown if these clinical symptoms are representative of this disease, as she is the only patient reported to date.
References
- 1. Chrzanowska KH et al. (2012) Nijmegen breakage syndrome. Orphanet J Rare Dis 7:13. doi: 10.1186/1750-1172-7-13. Full text on PubMed.
- 2. Varon R et al. (2000) Clinical ascertainment of Nijmegen breakage syndrome (NBS) and prevalence of the major mutation, 657del5, in three Slav populations. Eur J Hum Genet 8(11):900-902. Abstract on PubMed.
- 3. Varon R et al. (1999) Nijmegen Breakage Syndrome. Updated 2017 Feb 2. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 4. International Nijmegen Breakage Syndrome Study Group (2000) Nijmegen breakage syndrome. Arch Dis Child 82(5):400-406. Full text on PubMed.
- 5. Seemanov´ E et al. (2007) Cancer risk of heterozygotes with the NBN founder mutation. J Natl Cancer Inst 99(24):1875-1880. Full text from publisher.
- 6. Krüger L et al. (2006) Cancer incidence in Nijmegen breakage syndrome is modulated by the amount of a variant NBS protein. Carcinogenesis 28(1):107-111. Abstract on PubMed.
- 7. Distel L et al. (2003) Fatal toxicity following radio- and chemotherapy of medulloblastoma in a child with unrecognized Nijmegen breakage syndrome. Med Pediatr Oncol 41(4):44-48. Abstract on PubMed.
- 8. Bakhshi S et al. (2003) Medulloblastoma with adverse reaction to radiation therapy in Nijmegen breakage syndrome. J Pediatr Hematol Oncol 25(3):248-251. Abstract on PubMed.
- 9. Gregorek H et al. (2002) Heterogeneity of humoral immune abnormalities in children with Nijmegen breakage syndrome: an 8-year follow-up study in a single centre. Clin Exp Immunol 130(2):319-324. Full text on PubMed.
- 10. Salavoura K et al. (2008) Development of cancer in patients with primary immunodeficiencies. Anticancer Res 28(2B):1263-1269. Full text from publisher.
- 11. Seemanová E (1990) An increased risk for malignant neoplasms in heterozygotes for a syndrome of microcephaly, normal intelligence, growth retardation, remarkable facies, immunodeficiency and chromosomal instability. Mutat Res 238(3):321-324. Abstract on PubMed.
- 12. Borecka M et al. (2016) The c.657del5 variant in the NBN gene predisposes to pancreatic cancer. Gene 587(2):169-172. Abstract on PubMed.
- 13. Cybulski C et al. (2006) NBS1 is a prostate cancer susceptibility gene. Cancer Res 64(4):1215-1219. Full text from publisher.
- 14. Górski B et al. (2003) Germline 657del5 mutation in the NBS1 gene in breast cancer patients. Int J Cancer 106(3):379-381. Full text from publisher.
- 15. Steffen J et al. (2006) Increased risk of gastrointestinal lymphoma in carriers of the 657del5 NBS1 gene mutation. Int J Cancer 119(12):2970-2973. Full text from publisher.
- 15. Maraschio P et al. (2001) A novel mutation and novel features in Nijmegen breakage syndrome. J Med Genet 38(2):113-117. Full text on PubMed.
- 16. Chrzanowska KH et al. (2001) Atypical clinical picture of the Nijmegen breakage syndrome associated with developmental abnormalities of the brain. J Med Genet 38(1):1e3. doi: 10.1136/jmg.38.1.e3. Full text on PubMed.
- 17. Delia D et al. (2004) MRE11 mutations and impaired ATM-dependent responses in an Italian family with ataxia-telangiectasia-like disorder. Hum Mol Genet 13(18):2155-2163. Full text from publisher.
- 18. Stewart GS et al. (1999) The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99(16):577-587. Full text from publisher.
- 19. Matsumoto Y et al. (2011) Two unrelated patients with MRE11A mutations and Nijmegen breakage syndrome-like severe microcephaly. DNA Repair (Amst) 10(3):314-321. Abstract on PubMed.
- 20. Waltes R et al. (2009) Human RAD50 deficiency in a Nijmegen breakage syndrome-like disorder. Am J Hum Genet 84(5):605-616. Full text on PubMed.
- 21. Pasic S et al. (2013) Nijmegen breakage syndrome and chronic polyarthritis. Ital J Pediatr 39:59. doi: 10.1186/1824-7288-39-59. Full text on PubMed.