Subacute Sclerosing Panencephalitis (SSPE)
Subacute sclerosing panencephalitis (SSPE) is a progressive neurological complication of measles infection. SSPE is not caused by vaccination against measles.
The termsubacute means that the duration of a condition occurs over a period of time that is too long to be acute (like a cold or the flu) and too short to be chronic (like diabetes or asthma). SSPE usually lasts for weeks or months, though some cases have persisted for a few years. Sclerosing means that the disease causes scars/damages the brain. Finally, panencephalitis means that the entire brain can be affected. SSPE is also called subacute sclerosing leukoencephalitis, Dawson's encephalitis, and Van Bogaert encephalitis.
SSPE is caused exclusively by a long-term measles infection of the central nervous system. Again, it is not caused by vaccination against measles. The exact reason for the persistence of the virus in the brain is not clear, but it is currently thought that mutations in the virus and/or immature immune responses play a role (1-3). The measles virus can remain dormant in a person's nerve cells for years after infection before producing symptoms. However, the virus can eventually trigger an inflammatory response against infected cells, resulting in widespread destruction of neurons and myelin within the central nervous system (2, 3). The best way to prevent SSPE is to vaccinate children at early ages so as to reduce the chance that they will be infected with measles.
SSPE is increasingly rare in places with near-universal measles vaccination, but is much more common where measles transmission is still common (4, 5). SSPE has never been linked to a vaccine-strain of measles. For example, among SSPE patients who had been vaccinated against measles, the strain of virus isolated from their brain tissues and cerebrospinal fluid is invariably a wild-type strain 1-4).
Factors that increase the risk of developing SSPE include: measles infection before age five (and especially age 2), male gender, and (possibly) ethnicity. Papau New Guinea, parts of the Indian subcontinent, and the Middle East (Turkey and Pakistan) have very high rates of SSPE (4-6). This elevated risk may be due to low rates of vaccination. However, certain ethnic groups are overrepresented in SSPE registries of other nations (5, 7-9). The precise risk of developing SSPE is unknown, but it may occur in as many as 1 in 1700 measles cases among European or North American infants and toddlers who contract measles (4-9).
The CDC has many photos of people with measles.
Clinical information
The period between measles infection and the development of SSPE is months to decades, with most SSPE cases appearing 6 to 10 years after infection. Patients may not have a history of measles infection. This does NOT mean that SSPE develops in people who haven't had measles. Rather, it means that some patients were infected but not diagnosed. Studies that look for measles virus in the brains of people who died from SSPE find wild-type (disease-causing) virus. SSPE may also occur in people who have a been vaccinated: in general, these people were vaccinated after infection. Nearly all SSPE patients are less than 21 years of age when symptoms first appear. For example, we analyzed >700 SSPE cases in the literature; 607 reported age. Of this group, 98% were under age 21 when SSPE developed. In addition, males are at higher risk for SSPE. In our analysis of 732 cases for which sex was reported, 69% were male.
The onset of SSPE may be gradual over a period of weeks or months. Many patients initially have relatively minor, non-specific symptoms of neurological disease (2, 3, 5, 10). For example, many children often begin having difficulties in school and/or behavior problems that did not exist before. Visual disturbances and problems with fine motor skills such as writing may also appear. In general, SSPE patients may present with any combination of the following problems:
- Myoclonus (jerking movements which may cause falls or head drops; see video)
- Personality changes (uncharacteristic irritibility, aggression, psychological disturbances)
- Progressive cognitive impairment (memory lapses, confusion, poor school performance, etc.)
- Vision loss or deterioration, may be progressive or sudden
- Speech disturbances (slurring, loss of speech)
- Sleepiness or stupor
- Intention tremor or tics
- Changes to reflexes (Babinski's sign, increased deep tendon reflexes)
- Incontinence
- Loss of motor skills
- Muscle weakness or rigidity
Common problems in early stages of SSPE
Progression of SSPE (Jabbour Stages)
The initial signs of SSPE are mild relative to the severe and fatal problems that eventually develop (akinetic mutism/coma/death). Clinicians use a system that describes stages of disease during the course of the illness. This system helps with prognosis and helps them determine which interventions will be msot beneficial (see "Treatment and Prognosis" below). This system is ccalled the Jabbour Classification and is named after Dr. J.T. Jabbour, a neurologist who first proposed it.
The system uses the patient's level of impairment to determine how far the disease has progressed along its typical course (2, 10, 11). Progression from one stage to the next is highly idiosyncratic. For example, in the severest cases (about 10% of all cases), patients may progress from stage I to death in just a few months. Alternatively, a patient may move rapidly from one stage to the next and then stabilize for months or years. Some cases progress slowly overall, and, rarely, patients experience remission and regain lost skills. Patients may also have some functions which are relatively spared, resulting in a profile which includes features from multiple stages at once.
The table below lists the stages of SSPE. Information in this table was taken from reference 10 with minor changes to language, and is based on the original publication by Jabbour in 1969 (11).
Jabbour stages of SSPE |
||
Stage I |
IA. Behavioral, mental, and personality changes IB. Myoclonic spasms; focal and non-periodic |
|
Stage II |
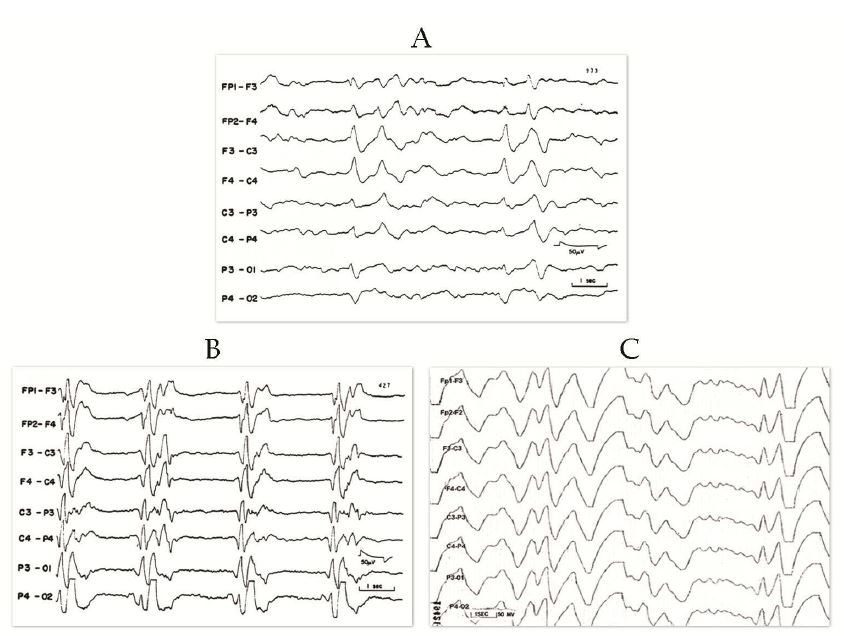
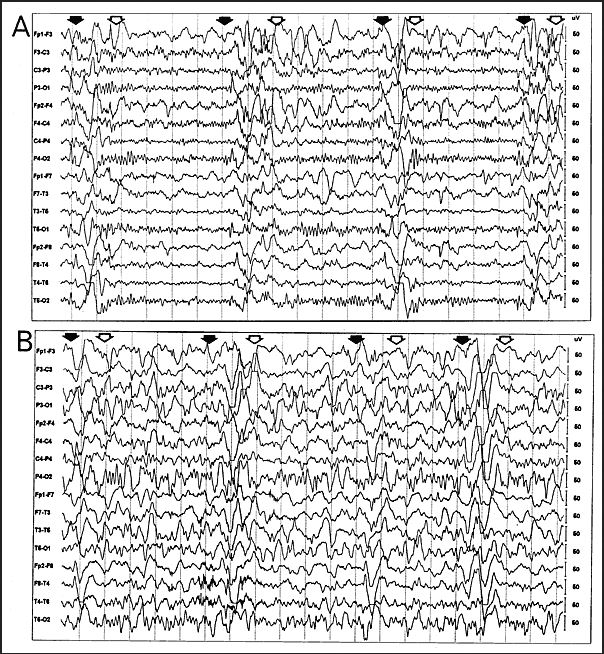
IIA. Additional mental deterioration IIA. Myoclonus becomes periodic and generalized IIA. Myoclonus may cause "drop" (falling) attacks when attempting to walk IIB. Apraxia, agnosia, language difficulties IIB. Motor dysfunction; ataxia, spasticity, inability to walk unaided IIB. EEG; periodic synchronous discharges coincident with myoclonic spasms (see video) |
|
Stage III |
IIIA. Patient speaks less IIIA. Vision deterioration IIIA. Seizures may occur IIIA. Myoclonic spasms every 3-5s IIIB. Patient is bedridden and has swalloing difficulties. IIIB. Choreiform movements, ballismus (both are involuntary movements) IIIB. EEG background delta |
|
Stage IV |
Myoclonus stops Akinetic mutism (coma/persistent vegetative state) EEG low voltage without periodic slow wave complexes |
Diagnosis, Testing, and Differential Diagnosis
SSPE should be considered in persons with dementia that progresses quickly, myoclonus, and seizures (2). The following major and minor criteria for diagnosis have also been established (2):
- Elevated measles antigens or antibodies from brain tissue or CSF
- Clinical history: Typical. Rapidly progressive, subacute nature
Atypical. Seizures, stage 1 longer than usual, patient is an infant or over age 21
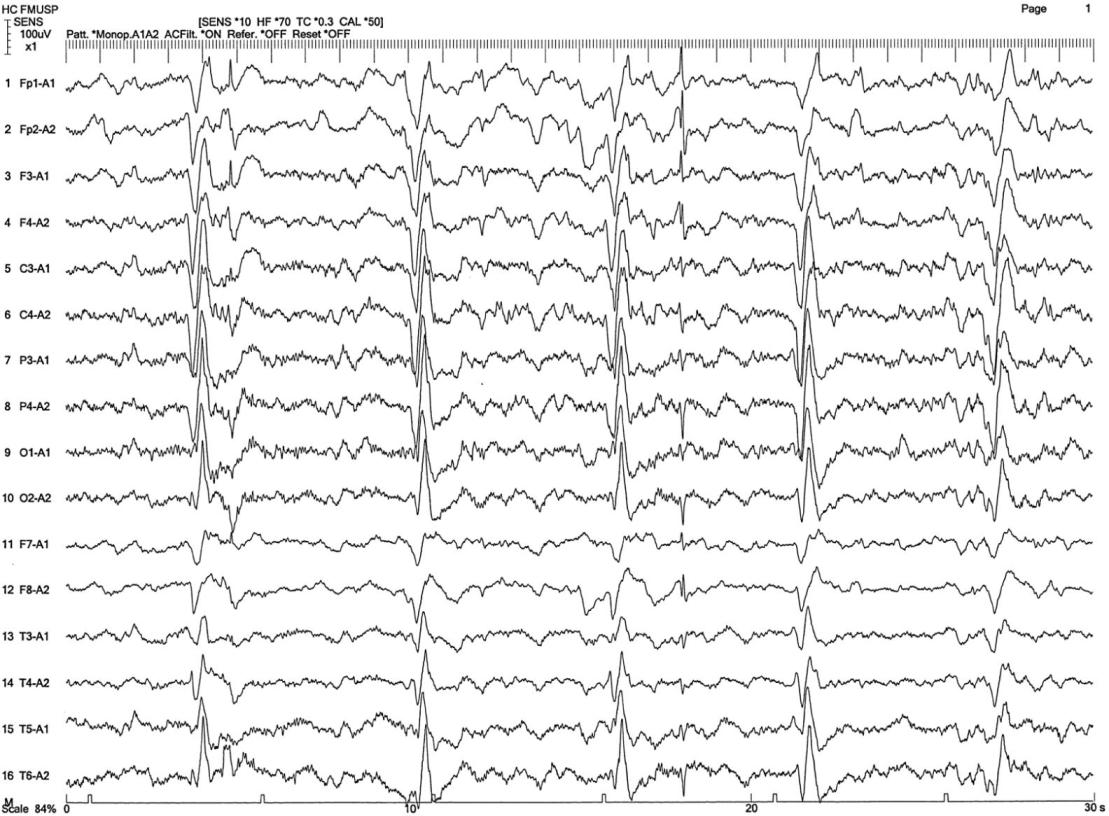
- EEG showing periodic complexes (see examples on this page)
- Increased IgG in CSF
- Brain biopsy shows deymelination, measles virus capsids, granulofilamentous inclusions in astrocytes, spongiosis, and other changes noted in reference 2
- Measles virus genome found in brain or CSF
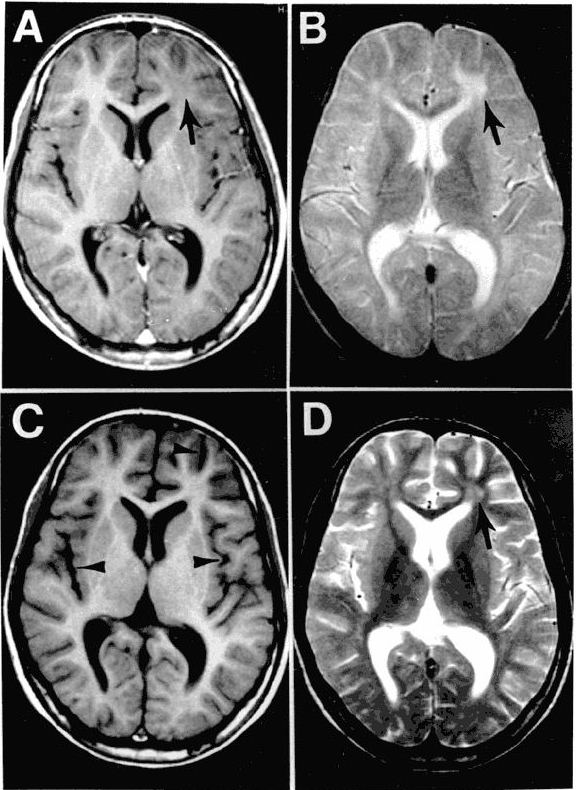
The EEG patterns that are characteristic of SSPE tend to appear at the same time as myoclonus (1, 10, 12). Before then, EEGs may be normal or display non-specific generalized slowing (1). In the early stages, diazepam may be used in initial stages to elicit the characteristic synchronous discharges (1). MRI in SSPE patients may or may not show brain lesions or atrophy, but this type of imaging may be useful in differential diagnosis. A typical progression of SSPE as observed in MRI images is shown below.
A number of other progressive neurological conditions should be considered in the differential diagnosis of SSPE.
Creutzfeldt-Jakob Disease (CJD) may present with disordered movement, deteriorating cognitive function, and EEG findings that are very similar to those in SSPE. Brain lesions visible in MRI are usually distinctive in CDJ, however, and measles antibody levels in CSF will not be elevated as they are in SSPE.
Progressive multifocal leukoencephalopathy (PML). PML's clinical features include ataxia, aphasia, and dementia, which also occur in SSPE. However, PML tends to occur in people who are immunocompromised due to other medical treatments or HIV infection. In addition, PML typically presents with hemiplagia (paralysis affecting one side of the body) and/or hemianopsia (loss of vision in one eye), which are not typical of SSPE.
Multiple sclerosis should be considered if elevated levels of measles antibodies cannot be isolated from CSF in a patient who presents with clinical symptoms of SSPE including significant demyelination.
Wilson's Disease (WD) may present with progressive neurological features. Serum copper and ceruloplasmin levels are unusually low in WD, however, and normal in SSPE. Liver disease and liver failure are common features of WD (13) that do not tend to occur in SSPE.
Hereditary hyperekplexia (HPX) may share myoclonus-like features with SSPE. However, HPX is typically seen in infants, while SSPE is not. In HPX, the startle-response is exaggerated and may appear to be a myoclonic spasm. Unlike myoclonus, this response is clearly evoked by a stimulus. Many individuals with HPX have an affected parent, which precludes SSPE.
Niemann-Pick disease type C (NPC) is a hereditary disorder that may resemble the onset of SSPE, but also involves the liver and includes vertical supranuclear gaze palsy not seen in typical cases of SSPE. NPC is identified by impaired cholesterol esterification and positive filipin staining in cultured fibroblasts. NPC is caused by defects in the genes NPC1 or (more rarely) NPC2.
Lafora-type progressive myoclonic epilepsy (LD) is an autosomal recessive disease that causes neurological deterioration and myoclonus in adolescents. Visual hallucinations and photosensitivity on EEG (high-voltage visual and somatosensory evoked potentials) are common in LD and not SSPE. LD may also be diagnosed via skin biopsy that shows the presence of Lafora bodies (starch-like polyglucosans). SSPE also usually results in demyelination, which is not observed in LD.
Other types of heritable myoclonic epilepsy may be related to POLG-related disorders such as Alpers-Huttenlocher syndrome, childhood myocerebrohepatopathy spectrum disorders, myoclonic epilepsy myopathy sensory ataxia, POLG-related ataxia neuropathy spectrum disorders, autosomal recessive progressive external ophthalmoplegia, or autosomal dominant progressive external ophthalmoplegia. Unlike SSPE, this family of inherited disorders may be accompanied by migraine, poor exercise tolerance, liver involvement (which may be worsened with valproate treatment to control seizures), cardiomyopathy and/or endocrine dysfunction. Tissue biopsies may reveal a respiratory chain defect and/or a defect of mitochondrial DNA (depletion or multiple deletions).
SSPE is still considered to be a fatal disease, with a terminal prognosis for 95% of patients. Death usually within usually occurs within 1-3 years of symptom onset (2). Remission, or halting of disease progression, occurs spontaneously in 5% of cases. Remission may be temporary, lasting weeks to years (2, 3), but patients are usually severely disabled even during this stage.
Care for SSPE patients is usually supportive and palliative. However, some therapies have been tried in a limited number of clinical trials. With currently used therapies, treatment of SSPE benefits at most 35% of patients (14, 15). Benefit has been defined as slower progression, stabilization of disease progression, improved survival, or (rarely) clinical improvement. To date, the most successful therapy for SSPE appears to be combination therapies including isoprinosine and interferon-alpha (14). Interferon-alpha is a protein with antiviral activity via enhancement of the cellular immune response, and isoprinosine is believed to disrupt viral replication.
Ribavarin has also been used for SSPE (16). This agent depletes infected cells of nucleotides and blocks RNA replication and protein synthesis thereby halting viral activity. Other pharmaceutical agents that have been used to treat SSPE are reviewed in reference 2.
In patients who show benefits with treatment, the treatment usually must be continued long-term in order to avoid relapse.
References
- 1. Gadoth N (2011) Subacute sclerosing pan-encephalitis (SSPE) - past and present. in Pathogenesis of Encephalitis, Dr. Daisuke Hayasaka (Ed.), ISBN: 978-953-307-741-3, InTech. Full text.
- 2. Gutierrez J et al (2010) Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol 52(10):901-907. FAbstract on PubMed. Full text on ResearchGate
- 3. Garg RK (2002) Review: Subacute sclerosing panencephalitis. Postgrad Med J 78:63-70. Full text on PubMed.
- 4. Bellini WJ et al. (2007) Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis 192(10):1686-1693. Full text from publisher.
- 5. Miller C et al. (2004) The epidemiology of subacute sclerosing panencephalitis in England and Wales 1990-2002. Arch Dis Child 89(12):1145-1148. Full text on PubMed.
- 6. Saha V et al. (1990) High incidence of subacute sclerosing panencephalitis in south India. Epidemiol Infect 104(1):151-156. Full text on PubMed.
- 7. Schönberger K et al. (2013) Epidemiology of subacute sclerosing panencephalitis (SSPE) in Germany from 2003 to 2009: a risk estimation. PLoS ONE 8(7):e68909. doi:10.1186/1471-2431-3-6. Full text on PubMed.
- 8. Prashanth LK, et al. (2007) Subacute sclerosing panencephalitis (SSPE): an insight into the diagnostic errors from a tertiary care university hospital. J Child Neurol 22(6):683-688. Abstract on PubMed.
- 9. Takasu T et al. (2003 ) A continuing high incidence of subacute sclerosing panencephalitis (SSPE) in the Eastern Highlands of Papua New Guinea. Epidemiol Infect 131(2):887-898. Full text on PubMed.
- 10. Campbell C et al. (2005) Subacute sclerosing panencephalitis: results of the Canadian Paediatric Surveillance Program and review of the literature. BMC Pediatr 5:47. doi: 10.1186/1471-2431-5-47 Full text on PubMed.
- 11. Jabbour JT et al. (1969) Subacute sclerosing panencephalitis. A multidisciplinary study of eight cases. JAMA 207(12):2248-2254. Citation on PubMed.
- 12. Silva-Júnior FP et al. (2013) The electroencephalographic signature of subacute sclerosing panencephalitis. Arq Neuro-psiquiatr 71(3):199. Full text from Scielo.
- 13. Weiss KH (1999) Wilson Disease. Updated May 16, 2013. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2016. Full text.
- 14. Gascon GG et al. (2003) A randomized treatment study of inosiplex versus combined inosiplex and intraventricular interferon-alpha in subacute sclerosing panencephalitis (SSPE): international multicenter study. J Child Neurol 18:819-827. Abstract on PubMed.
- 15. Dyken PR et al. (1982) Long-term follow-up of patients with subacute sclerosing panencephalitis treated with inosiplex. Ann Neurol 11:359-364. Abstract on PubMed.
- 16. Hosoya M et al. (2004) Pharmacokinetics and effects of ribavirin following intraventricular administration for treatment of subacute sclerosing panencephalitis. Antimicrob Agents Chemother 48(12):4631-4635. Full text on PubMed.