Smith-Lemli-Opitz syndrome (SLOS)
Smith-Lemli-Opitz syndrome, or SLOS, is named for three doctors who described it in 1964 (1). SLOS is an inherited condition that affects most body systems (see Clinical Features below). It has also been called RSH syndrome (for the initials of the patients described in the 1964 paper). SLOS is sometimes described as a malformation syndrome because it causes parts of the body to form incorrectly.
SLOS is a very rare disorder that seems to occur most often in people of northern and central European origin (2). It appears to be very rare among people of Asian and African origin, although it has been documented in these groups (3-8), and others. Many studies have tried to estimate how often it occurs, but estimates vary. For example, a study in Canada found that annual incidence of SLOS was 1 in 17,000 to 1 in 29,000 births (9). Similarly, incidence in Slovakia was estimated at 1 in 15,000 to 1 in 20,000 births (10). Alternatively, incidence in the UK was estimated at 1 per 60,000-67,000 male births (11). The rate is presumably much lower in Japan (3). Reference 2 has a detailed discussion of this topic.
Clinical information
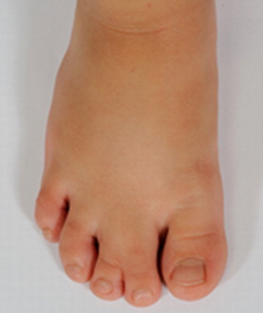
SLOS affects many body systems. Its most characteristic features are low blood cholesterol, fusion of the second and third toes (see photo), failure to thrive in infancy (with or without feeding difficulties), a small head, and developmental delays including intellectual disability. Because of the very large number of clinical features that occur in SLOS, we have organized our list below by system affected.
SLOS is often confused with other conditions (see Differential Diagnosis). As a result, no diagnosis is definitive until it has been confirmed biochemically (see Diagnosis). The list of clinical features below is not complete, and the problems that happen vary from patient to patient.
- The fetus may be less active than would be expected
- Malformations may be visible on an ultrasound
- Poor growth (low birthweight and birth length)
- Breathing problems at birth
- Breech birth
- Delayed development of any gross motor skills (head control, sitting, walking, etc.)
- Feeding problems, including poor sucking, and vomiting/spitting up
- Failure to thrive (poor weight gain in an infant/child)
- Intellectual disability/very low IQ
- Delayed speech development
- Very short stature
- Autism or autism-like behaviors (insistence on routines, poor eye contact, hand-flapping, etc.)
- A tendency to arch the back and forcefully throw oneself backward ("opisthokinesis")
- Self-injurious behavior (head-banging, may scratch himself or herself)
- Sleep abnormalities (trouble falling asleep, wakening at night, etc.)
- Sleeps less than expected (may sleep for only 2-3 hours at night)
- Temper tantrums or other aggressive behavior
- Patients may be affectionate and loving
- Floppiness (hypotonia) as an infant; may change to stiffness (hypertonia) with time
- Abnormal curvature of the spine (scoliosis, kyphosis/rounded back)
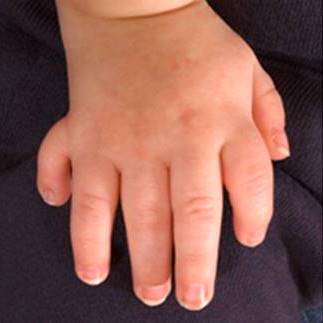
- Short thumbs that may be abnormally positioned (low on the hand)
- Syndactyly of second and third toes (toes fused; see photo)
- Curvature of a finger, often the pinky
- Extra fingers or toes
- Genitals may have male and female features, especially in boys (but chromosomes are X,Y)
- Pyloric stenosis (the opening from the stomach to the small intestine is very narrow)
- Hypospadias (hole in penis is not at the end of the penis)
- Undescended testicles (cryptorchidism)
- Very small genitals (especially in boys)
- Girls' genitals are often normal
- Photosensitivity (sunburn that develops within minutes of sun exposure)
- Abnormalities of brain anatomy (visible on a scan)
- Frequent infections (especially ear infections)
- Susceptibility to pneumonia
- Abnormal heart anatomy
- Kidney abnormalities
- High blood pressure
- Cataracts
Common clinical features of SLOS
Before and at birth
Growth & Development
Behavior
Muscles and skeleton
Genitals
Other
Unfortunately, this list is not complete. A comprehensive description of the clinical features of SLOS may be found in reviews (2, 12).
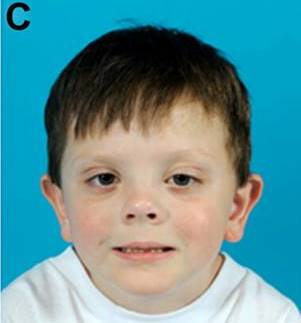
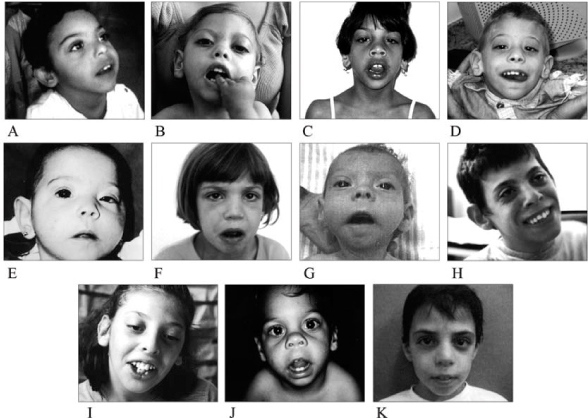
In addition to the clinical features listed above, many people with SLOS have similar facial features. The most common ones are listed below. These features may not be present in every patient, they may be subtle, and they may become less obvious with time (see photos on this page).
- Epicanthal folds (skin covering the inner corners of eyelids)
- Rotated ears (tops point toward the back of the head)
- Strabismus (eyes not aligned properly)
- Receding/small/underdeveloped jaw
- Ptosis (droopy upper eyelid)
- Upturned nose
- Low-set ears
- Short nose
Facial features of SLOS
Cause
SLOS is caused by a mutation in the gene DHCR7, and is an autosomal recessive disorder. This term means that it is caused by a mutation in a gene not located on an X or Y chromosome, and that a baby will have SLOS only if each parent contributes a copy of the mutated gene.
DHCR7 has an important role in making cholesterol, and when it is damaged, the body may not be able to make enough of it. This situation creates problems even though cholesterol can easily be obtained through diet. This is because cholesterol is important for brain function, yet can't pass through a group of cells that make up the blood-brain barrier. This barrier protects the brain from many toxic substances by keeping them out. However, the brain needs cholesterol, so it must therefore make it itself. In SLOS, this process is impaired. Low levels of cholesterol in the brain appear to be responsible for some of the developmental problems in SLOS (13).
Diagnosis & Managment
Although the clinical features of SLOS vary, the syndrome should be considered when the following are present together (13):
- Fusion/syndactyly of the second and third toes
- Other malformations
- Behavioral disturbances
- Developmental problems, especially low IQ
- Failure to thrive
Any suspected diagnosis of SLOS must be confirmed with biochemical tests and/or gene sequencing. Sequencing looks for mutations in the gene DHCR7, while biochemical tests measure blood levels of a substance called 7-DHC.
The gene DHCR7 makes an enzyme that transforms a subtance called 7-DHC into cholesterol. Most people have very little 7-DHC in their blood because it is made and then immediately transformed. This fact makes it useful for testing for SLOS. In most situations, its presence above baseline levels is a strong indicator that a person has SLOS.
Standard tests for total cholesterol used in many hospitals measure 7-DHC in addition to completely formed cholesterol (14). As a result, cholesterol levels in a minority of SLOS patients may seem to be normal. This fact should be considered in any patient suspected of having SLOS who has normal cholsterol test results. Thus, normal results from standard lab tests should not be used to rule out SLOS (15); rather, gene sequencing should be used. An excellent review discusses pitfalls of measuring cholesterol in SLOS (16). See Differential Diagnosis below for information on conditions that may increase levels of 7-DHC in the blood.
There is no cure for SLOS. Cholesterol supplements and increased dietary intake of cholesterol are used to manage it. While supplementary cholesterol does not cross the blood-brain barrier and therefore is unlikely to help with intellectual disabilities and behavioral problems, it does appear to help with a variety of other symptoms. For example, several small studies have reported that it improved behavior (reviewed in 2), growth, muscle tone, and strength (reviewed in 13). Extra dietary cholesterol may also diminish photosensitivity (17, 18). It also appears to reduce the amount of 7-DHC in the blood, fact that should be considered if an undiagnosed patient who eats a high-cholesterol diet is tested for SLOS.
A severity score has been developed for SLOS (see reference 2). This score characterizes a case of SLOS as severe, moderate, and mild categories, which can help healthcare providers understand some aspects of SLOS. However, its overall focus is on malformations, and it therefore does not consider a person's level of intellectual disability, behavioral problems, or feeding difficulties.
Differential Diagnosis
Noonan syndrome (NS). NS can cause intellectual disabilities, short stature, heart problems, and undescended testicles. These problems also occur in SLOS. Differences between NS and SLOS can help distinguish them. For example, a large head (macrocephaly) is common in NS but does not generally occur in SLOS, which is associated with a small head (microcephaly). Other features that may help distinguish the two conditions include a sunken chest (common in NS but not in SLOS), and ambiguous genitalia (occurs in a majority of SLOS patients, but does not seem to be a problem in NS, beyond undescended testicles). Genetic testing and testing for 7-DHC can make a diagnosis definitive. NS is associated with mutations in three genes: PTPN1, SOS1, and BRAF.
Haloperidol use. Haloperidol (aka Haldol) is a psychotropic drug used as in long-term treatment of schizophrenia. It is also used in a variety of other conditions including severe anxiety and some severe behavior problems. Haloperidol may elevate blood levels of 7-DHC, which can cause a false positive result in a test for SLOS (12).
Cerebrotendinous xanthomatosis (CTX). CTX can cause elevations of 7-DHC, and patients may develop cataracts, which are also found in a minority of SLOS patients. People with CTX tend to suffer from cholestatic liver disease, which, again, also happen in severe cases of SLOS (2). In addition, diarrhea in infancy in CTX can lead to failure to thrive, and cognitive and psychiatric problems may occur. However, in spite of these common symptoms, CTS does not resemble SLOS in most other ways. For example, CTX patients do not have a higher rate of malformations, and many CTX patients have xanthomas, which are fatty nodules that often occur along tendons. Xanthomas are not associated with SLOS.
CTX can be distinguished from SLOS by lab testing. See reference 19 for details.
Other conditions in the differential diagnosis for SLOS are listed in reference 14, which is freely available on the internet.
References
- 1. Smith DW Lemli L & Opitz JM (1964) A newly recognized syndrome of multiple congenital anomalies. J Pediatr 64(2):210-217. Abstract on PubMed.
- 2. Nowaczyk MJ & Irons MB (2012) Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet 160C(4):250-262. Abstract on PubMed.
- 3. Tsukahara M et al. (1998) Smith-Lemli-Opitz syndrome in Japan. Am J Med Genet 75(1):118-119. Abstract on PubMed.
- 4. Matsumoto Y et al. (2005) R352Q mutation of the DHCR7 gene is common among Japanese Smith-Lemli-Opitz syndrome patients. J Hum Genet 50(7):353-356. Abstract on PubMed.
- 5. Verma IC & Ghai OP (1971) Smith-Lemlie-Opitz syndrome in an Indian child. Indian Pediatr 8(5):221-222. Abstract on PubMed.
- 6. Hanissian AS & Summitt RL (1969) Smith-Lemli-Opitz syndrome in a negro child. J Pediatr 74(2):303-305. Abstract on PubMed.
- 7. Al-Owain M et al. (2012) Smith-Lemli-Opitz syndrome among Arabs. Clin Genet 82(2):165-172. Abstract on PubMed.
- 8. Scalco FB et al. (2006) Smith-Lemli-Opitz syndrome: clinical and biochemical findings in Brazilian patients Genet Mol Biol 29(3):429-436. Full text on Scielo.
- 9. Waye JS et al. (2002) Smith-Lemli-Opitz syndrome: carrier frequency and spectrum of DHCR7 mutations in Canada. J Med Genet 39(6):e31. doi: 10.1136/jmg.39.6.e31 Full text on PubMed.
- 10. Bzdúch V et al. (2000) Incidence of Smith-Lemli-Opitz syndrome in Slovakia. Am J Med Genet 90(3):260. Abstract on PubMed.
- 11. Ryan AK et al. (1998) Smith-Lemli-Opitz syndrome: a variable clinical and biochemical phenotype. J Med Genet 35(7):558-565. Abstract on PubMed.
- 12. Kelley RI & Hennekam RC (2000) The Smith-Lemli-Opitz syndrome. J Med Genet 37(5):321-325. Full tex on PubMed.
- 13. Porter FD & Herman GE (2011) Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res 52(1):6-34. Full text on PubMed.
- 14. Nowaczyk MJM (1998) Smith-Lemli-Opitz Syndrome. Updated June 20, 2013. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2017. Full text.
- 15. Jezela-Stanek A et al. (2008) Mild Smith-Lemli-Opitz syndrome: further delineation of 5 Polish cases and review of the literature. Eur J Med Genet 51(2):124-140. Abstract on PubMed.
- 16. Jira PE et al. (1997) Pitfalls in measuring plasma cholesterol in the Smith-Lemli-Opitz syndrome. Clin Chem 43(1):129-133. Full text from publisher.
- 17. Elias ER et al. (1997) Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS). Am J Med Genet 68(3):305-310. Abstract on PubMed.
- 18. Azurdia RM et al. (2001) Cholesterol supplementation objectively reduces photosensitivity in the Smith-Lemli-Opitz syndrome. Br J Dermatol 144(1):143-145. Abstract on PubMed.
- 19. Federico A et al. (2003) Cerebrotendinous Xanthomatosis. Updated April 14, 2016. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2017. Full text.