Menkes Disease (MD)
Menkes disease is a member of a small group of conditions caused by mutations in a gene called copper-transporting ATPase gene (ATP7A). As its name implies, ATP7A has a role in regulating copper levels. Copper is an essential mineral. When it is absent or deficient, a person may have problems that resemble anemia, neutropenia (a low number of neutrophils; a type of white blood cell that helps fight infections), bone abnormalities including osteoporosis, abnormally pale and other problems.
The other diseases in this group are occipital horn syndrome (OHS) and ATP7A-related distal motor neuropathy (DMN).
Clinical information
Menkes disease is a serious condition that primarily affects males. Patients with this condition cannot transport copper because of mutations in ATP7A. As a result, the levels of copper in their blood and brain are low to non-existent. Ceruloplasmin (a protein that helps transport copper) levels are also low. Because they have so little copper, patients with MD experience progressive neurodegeneration (nervous system problems that get worse), abnormalities of connective tissue, and hair that is brittle, steely, and coarse. MD can be treated with parenteral copper-histidine supplementation (oral supplements become trapped in the intestine and are ineffective). It is most treatable if therapy begins very early in infancy (1, 2).
Patients with MD suffer from seizures, intellectual disabilities, and developmental disorders. At birth, they may appear to be neurologically normal. Beginning around 2 months of age, they become hypotonic, (floppy) and develop other problems. Blood testing typically shows decreased serum copper and decreased serum ceruloplasmin. However, levels in affected newborns may overlap with those in healthy newborns (see Diagnosis and Testing, below).
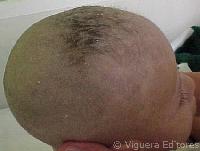
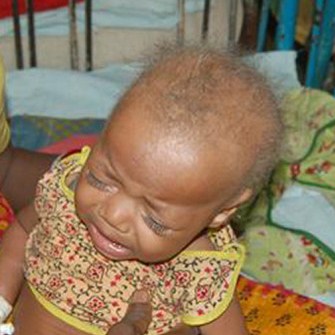
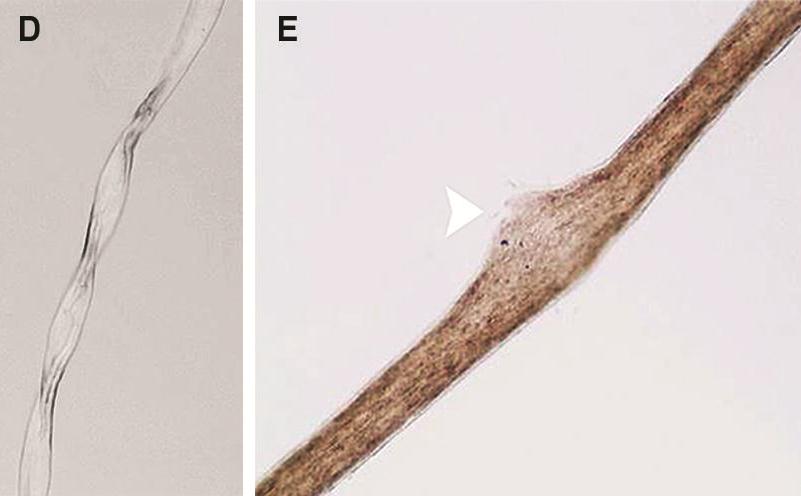
The hair of MD patients often has pili torti, monilethrix, and/or trichorrhexis nodosa (paintbrush hair; see photos below). Although the hair in newborns with MD may appear abnormal, pili torti may not be appear until infants are 2-4 months old. Neurologically, newborns with Menkes disease generally appear healthy. Beginning at 2-4 months, the following signs may appear:
- Seizures
- EEG abnormalities
- Brain abnormalities on MRI (see photos below)
- Tortuous blood vessels in the brain (see photos below)
- Acquired microcephaly (head growth slows and falls below the 2nd-3rd percentile)
- X-ray abnormalities, including Wormian bones in the skull, bone spurs (see photo below), and rib abnormalities including fractures
- Osteoporosis
- Bladder diverticula
- Chubby cheeks
- Irritability
- Loss of skills
Signs of Menkes disease
MD has been called Menkes kinky hair syndrome, steely hair disease, and trichopoliodystrophy. It is very rare. Various studies estimated an incidence of 1 person in 298,000 in 5 countries in western Europe (3). Similarly, estimates for incidence in in Japan are 1 in 360,000 (4). Its incidence in Australia was estimated in 1971 at 1 in 40,000 births based on 7 cases during a 4-year period there (5). This higher incidence may have been due to a founder effect. Overall, MD appears to occur in all ethnic groups. Reference number 2 is a thorough review of this condition. Clinicians may also wish to read a description of EEG patterns in MD (6).
Menkes disease is caused by mutations in the gene ATP7A. This gene is located on the X chromosome, which means that the disease is passed from a mother to a son (girls get two copies of the X chromosome, and the healthy copy of ATP7A that they get from their fathers stops them from getting the disease). However, some female carriers show some signs related to Menkes disease. These signs include hair abnormalities such as pili torti and skin abnormalities (7). In addition, there have been reports of females with Menkes disease, diagnosis was confirmed by genetic analysis (4,8,9). These patients came from different backgrounds (Japan, Italy, United States). Thus, although it is unlikely that Menkes will occur in a female, it is not impossible, and the diagnosis should not be excluded in a girl showing the symptoms noted above.
The more serious problems associated with MD are often not become obvious until an infant is 2-4 months old. This fact complicates the situation for patients, given that treatment is most effective if begun in the newborn period. Signs that may be present at birth include the following:
- Premature labor and delivery
- Low birthweight
- Loose, redundant skin (the child may appear to have too much skin): especially on the neck, armpits, and trunk
- Hypothermia (low body temperature)
- Jaundice
- Low blood sugar
- Cephalohematoma (a collection of blood under a skull bone; makes the skull bulge)
- Pale, hypopigmented hair (very common, but not necessarily at birth; may darken later)
Signs that may be present at birth
As noted above, serum copper levels in affected infants are low, but can overlap with levels in healthy infants. Fortunately, advances in diagnostics have shown that the ratio of plasma dopamine to norepinephrine is higher in affected newborns compared to unaffected newborns (1,2). The levels of these two molecules cannot be examined singly; they must be assessed as a ratio. The ratio of dihydroxyphenylacetic acid to dihydroxyphenylglycol is also a reliable test for MD. See references 1 and 2 for details.
MD is caused by mutations in ATP7, a gene that produces a copper transport protein. ATP7 is located on the X chromosome, which is why MD affects primarily males. Some females also have symptoms, though they tend to be mild (10).
One other condition in the ATP7A group are part of the differential diagnosis for Menkes syndrome. For example, occipital horn syndrome (OHS) is viewed by many as a mild form of Menkes disease (1), although it does have distiguishing features. The most important one is the presence of occipital horns on radiographic images. Occipatal horns are bony wedge-shaped calcium deposits in tendons that attach to the occipital bone, which is the lower bone at the back of the skull. They may be felt in some patients, and are generally visible radiographically. They may not occur until after age 10 (1). In addition, unlike Menkes disease patients, OSH patients have some capability to transport copper. Serum copper levels in this group may be low or normal, while levels of catecholamine are abnormal in the serum and CSF. Their ability to transport copper spares them from the severe neurodegeneration that afflicts MD patients. However, the ATP7A mutation gives them generalized muscle weakness, and mild dysautonomia (in this case, a tendency to faint, low blood pressure upon standing, and chronic diarrhea; 1). Like MD patients, they tend to have coarse hair and loose joints. In part because it is a relatively mild condition, OSH does not usually become evident until the toddler years or later (ages 3-10; 1). In contrast, MD generally manifests during the neonatal period or up to age 1. Very few OHS patients have been described in the literature, and the course of the disease has not been well-characterized.
The other condition in the ATP7A group is called ATP7A-related distal motor neuropathy (DMN). DMN is clinically and biochemically distinct from MD and OHS. Onset occurs in adults, and DMN most closely resembles Charcot-Marie-Tooth disease (CMT). DMN was described relatively recently. To date, no patients have had hair, skin or joint abnormalities, nor have they been found to have low serum copper, or abnormal plasma catecholamine (1).
Menkes syndrome should be also distinguished from child abuse (rib fractures may lead to an erroneous suspicion that the infant was abused), viral encephalities, tyrosinemia, biotin deficiency, trichothiodystrophy (hair abnormalities and intellectual disabilities are similar), argininosuccinic aciduria & citrullinemia, and Politt syndrome.
References
- 1. Kaler SG et al. (2008) Neonatal Diagnosis and Treatment of Menkes Disease. N Engl J Med 358:605-614. Full text on PubMed.
- 2. Goldstein DS et al. (2009) Clinical outcomes in Menkes disease patients with a copper-responsive ATP7A mutation. Neurochem Res 34(8):1364-1368. Full text on PubMed.
- 3. Tønneson T et al. (1991) Incidence of Menkes disease. Hum Genet 86(4):408-410. Abstract on PubMed.
- 4. Gu YH et al. (2005) A survey of Japanese patients with Menkes disease from 1990 to 2003: Incidence and early signs before typical symptomatic onset, pointing the way to earlier diagnosis. J Inherit Metab Dis 28:473-478. Abstract on PubMed.
- 5. Danks QC et al. (1971) Is Menkes' syndrome a heritable disorder of connective tissue?. Lancet 2(7733):1089. Abstract on PubMed.
- 6. Bahi-Buisson N et al. (2006) Epilepsy in Menkes disease: analysis of clinical stages. Epilepsia 47(2):380-386. Abstract on PubMed.
- 7. Moore CM & Howell RR (1985) Ectodermal manifestations in Menkes disease. Clin Genet 28:532-540. Abstract on PubMed.
- 8. Kapur et al. (1987) Menkes syndrome in a girl with X-autosome translocation. Am J Med Gen 26:503-510. Abstract on PubMed.
- 9. Cosimo QC et al. (2011) Kinky Hair, Kinky Vessels, and Bladder Diverticula in Menkes Disease. J Neuroimaging 21:e114-e116. Abstract on PubMed.
- 10. Møller LB et al (2012) Clinical expression of Menkes disease in females with normal karyotype. Orphanet J Rare Dis 7:6. Full text on PubMed.
- 11. Datta AK et al. (2008) Menkes kinky hair disease: A case report. Cases J 1(1):158. Full text on PubMed.
- 12. Mendonça do Rego JI et al. (2014) Imaging features that allow for the recognition of Menkes disease. Arq Neuro-Psiquiatr 72(5):396. Full text from Scielo.
- 13. Venta-Sobero JA et al. (2004) Síndrome de West como manifestación epiléptica de enfermedad de Menkes. Presentación de dos casos. Rev Neurol 39:133-136. Full text from publisher.
- 14. Jain P et al. (2014) Menkes disease - an important cause of early onset refractory seizures. J Pediatr Neurosci 9(1):11-16. Full text on PubMed.