Crouzon syndrome
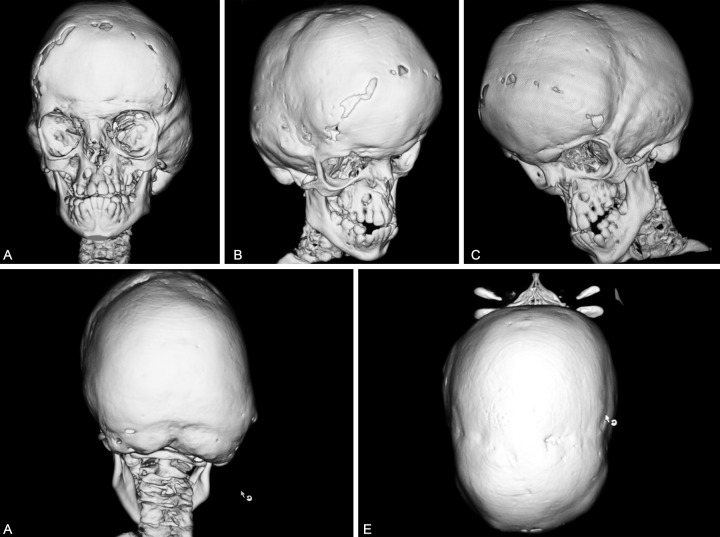
Crouzon syndrome (CS) is member of a group of disorders involving craniosynostosis. This term means that at least one of a person's skull bones fuses prematurely. The problem is often noted at birth, but it may be picked up on an ultrasound or it may not become evident until well after birth. Premature fusion of skull bones restricts skull growth, causing it to expand unevenly to accomodate brain growth.
When only one brain suture is closed prematurely, a person is said to have isolated craniosynostosis. This condition is relatively common, occuring in roughly 3-5 per 10,000 births or even more frequently (1, 2). Severity varies widely, with some cases being severe and others being barely noticeable. The causes for isolated craniosynostosis are not fully understood, but hereditary/genetic components are involved (1, 3). Possible risk factors for craniosynostosis include, but are not limited to, the mother's age and race (more common among older women and whites); 4), male sex (4), and heavy maternal smoking, especially after the first trimester (5).
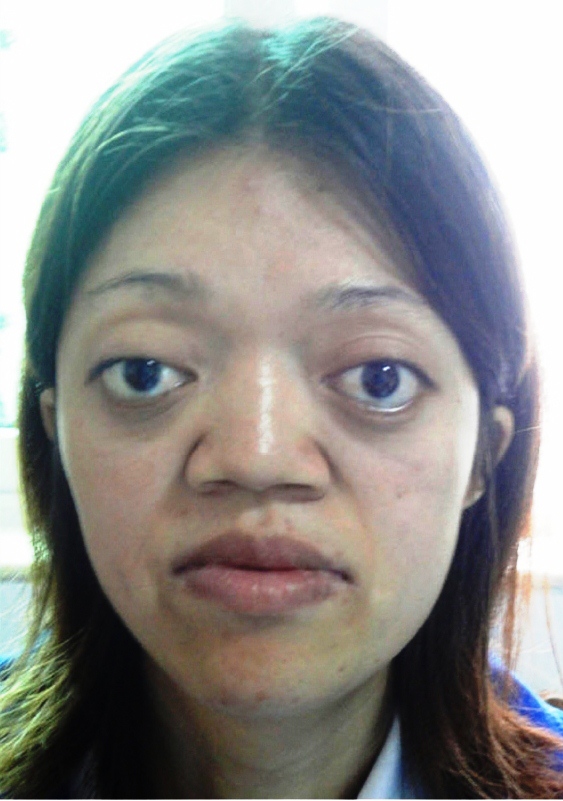
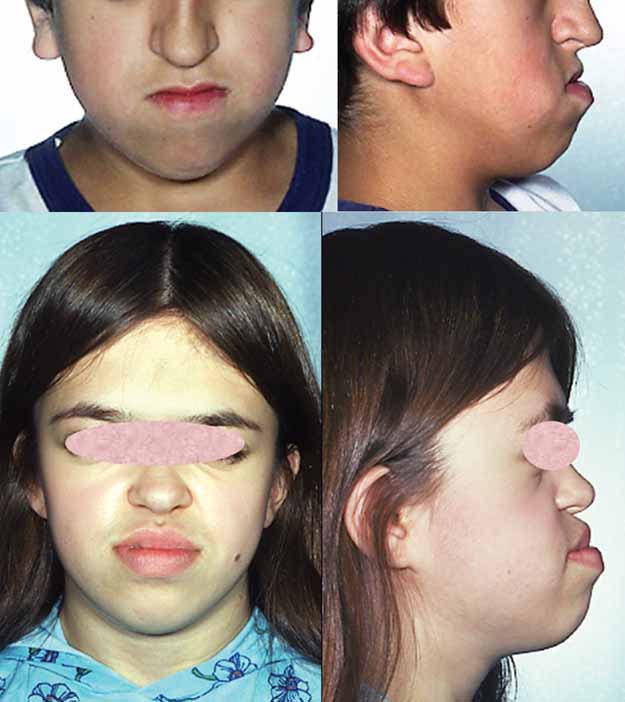
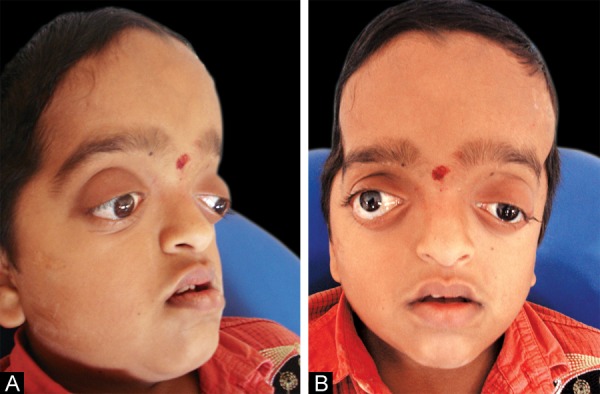
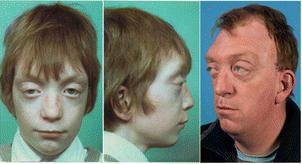
In addition to isolated craniosynostosis, there are also a number of craniosynostosis syndromes. As the name implies, the syndromic forms of craniosynostosis involve problems in a variety of body systems. The hands and/or feet are often affected, a fact that implies that skull and hand/foot development share some type of common mechanism. Crouzon syndrome is a member of a group named FGFR-related craniosynostosis syndromes. All members of this group are caused by mutations in the genes FGFR1, FGFR2, or FGFR3. In addition to Crouzon syndrome, the group includes Crouzon syndrome with acanthosis nigricans, Pfeiffer syndrome, Apert syndrome, Muenke syndrome, Jackson-Weiss syndrome, Beare-Stevenson syndrome, and FGFR2-related isolated coronal synostosis. Generally speaking, skull abnormalities in these syndromes are observed in the newborn period or before, although some cases may not be noted until later infancy or afterward. In addition to having abnormally shaped skulls, people with these syndromes tend to have wideset eyes that protrude outward (proptosis), an underdeveloped midface (hypoplasia; best seen in profile; see photos on this page), a small nose that may be beaked, and a highly arched palate or a cleft palate (see photo on this page; 6, 7). Intellectual disability, common in other craniosynostoses, is uncommon in CS (6, 7).
Crouzon syndrome affects males and females equally. It has also been seen in all races and ethnic groups around the world. In our analysis of 175 published cases of CS, 48% were male and 52% were female. We found reports describing people of European descent, as well as descriptions of Asians (both eastern and western Asia), Latinos, sub-Saharan Africans and African-Americans, Arabs, and others.
Estimates about the prevalence of Crouzon syndrome vary. One widely accepted figure is 16.5 cases per million at birth, or roughly one per 60,000 (8). Other estimates indicate rates as high as one per 35,000 births (6).
Crouzon syndrome is also called Type I Craniofacial Dysostosis, Crouzon Type Craniostenosis, and Crouzon Craniofacial Dysostosis (6).
Clinical information
The cardinal features of CS are orbital proptosis (protrusion of the eyes), which may be severe, and craniosynostosis. In radiographs, the inner table of the skull may resemble hammered or beaten metal (see photos on this page). This unusual feature is called copper beaten skull or digital impressions. It is caused (in part at least) by raised intracranial pressure (ICP; 9), which, in turn, can be caused by craniosynostosis. Raised ICP is associated with morning headaches that may get worse with time, vomiting, and the onset of developmental delay, but these signs are not specific or sensitive enough to diagnose the problem (10). The current standard for diagnosis is direct intraparenchymal monitoring, which is an invasive technique. Noninvasive methods are described in reference 10. Early diagnosis is important so that intracranial pressure can be monitored closely during childhood in case surgical interventions are needed (11, 12).
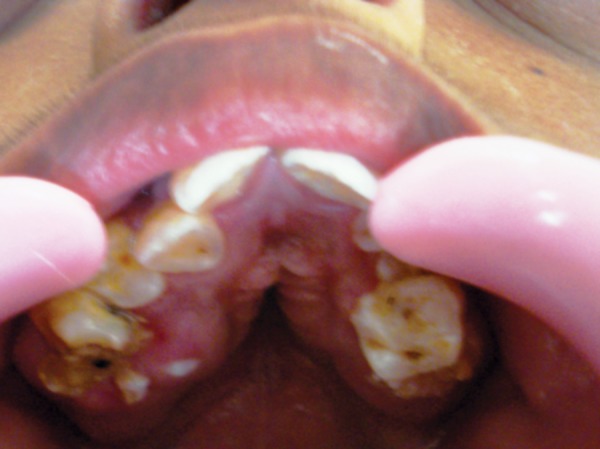
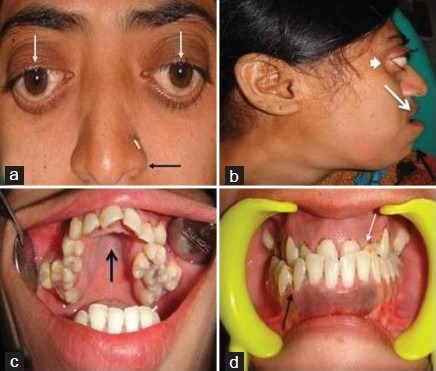
Midface hypoplasia is also very common in patients with CS. This term means that a portion of the middle part of the face is underdeveloped. When viewed in profile, people with midface hypoplasia often appear to have a convex facial structure (see photos on this page). Dental abnormalities are also very common, as is a highly arched or cleft palate; cleft lip occurs in a small minority of cases. Dental abnormalities may require interventions during childhood (13).
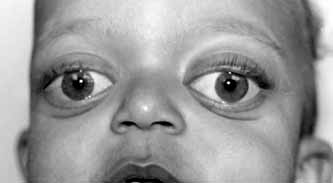
Proptosis, or bulging eyes, is a common characteristic (6). It may not be noticeable at birth, and can develop or worsen over the first years of life (11, 14). Proptosis in Crouzon syndrome occurs as the result of shallow eye sockets (see photos below; 15). It can become severe and may impair vision (14).
As noted above, most people with Crouzon syndrome do not have an intellectual disability. However, intellectual disability should not be used to exclude a diagnosis of Crouzon syndrome, as it is commonly reported in a small number of patients (11, 12, 14). In our survey of the literature, it was reported in 11% of 151 patients for whom data was available.
Syndactyly of the hands and feet may occur in CS, as in the other craniosynostosis syndromes, but is uncommon in CS and generally much milder than in other syndromes (11). Sometimes shortened bones in the fingers and thumb can be observed in radiographs, but the malformation may otherwise go unnoticed (6).
The two lists below provide common clinical features of Crouzon syndrome; the first list has general features, and the second one has common facial features. Because CS overlaps with other craniosynostosis syndromes, laboratory testing may be required to make a definitive diagnosis.
- Craniosynostosis (premature fusion of the skull bones, accompanied by unusual skull shape
- Dental abnormalities (crowding, abnormally positioned teeth, etc.)
- Beaten-copper appearance of skull in radiographs (see photos)
- Highly arched palate (see photos below)
- Elevated intracranial pressure
- Bulging anterior fontanel
- Hearing loss
- Vision loss
Common clinical features of Crouzon syndrome
Many of the following facial features are shared with other members of the FGFR-related craniosynostosis syndromes, including Apert syndrome and Pfeiffer syndrome:
- Midface hypoplasia (the cheekbones, upper jaw, and eye sockets are underdeveloped)
- Downslanting palpebral fissures (see photo at top right)
- Prognathism (jutting of lower jaw)
- Strabismus (eyes not aligned)
- Hypertelorism/wideset eyes
- Proptosis/protruding eyes
- Facial asymmetry
- Large forehead
- Beaked nose
- Lowset ears
Diagnosis and Testing
An early diagnosis of CS is important to avoid damage to brain function vision, which may result from raised ICP. CS should generally be suspected in an infant or older person who presents with craniosynostosis, proptosis, and midface hypoplasia without severe syndactyly (the latter is common in Apert syndrome but not in CS; 6, 7, 11, 12). These abnormalities occur nearly universally in CS.
CS is caused by a variety of mutations in the gene FGFR2. It is an autosomal dominant disorder. This term means that only one copy of a mutated gene is needed to cause the disorder. Roughly 70% of CS patients have an affected parent. A person affected by CS has a 50% chance of passing the trait to future offspring. Some cases of CS are sporadic, meaning that they arise when a mutation in FGFR2 occurs randomly and for reasons that are not known. In those cases, recurrence of CS is not likely in future offspring of the same parents (but their affected child may pass the syndrome on to future children). Advanced paternal age has been found to be a risk factor for CS and other members of this syndrome group (16-18).
In some cases, the same mutations in FGFR2 can cause Apert syndrome, Pfeiffer syndrome, Jackson-Weiss syndrome, and/or Crouzon syndrome with acanthosis nigricans (6, 7, 19). Researchers do not currently understand why the same mutations can lead to different syndromes. Thus, genetic testing may not be as useful for establishing a diagnosis as in some other genetically-acquired rare diseases. For additional details about the genetics of the FGFR-related syndromes and testing, see reference 6.
Differential Diagnosis
The differential diagnosis for CS includes certain other craniosynostosis syndromes.
Crouzonodermoskeletal syndrome. This syndrome was formerly called Crouzon syndrome with acanthosis nigricans (CAN). It was renamed to reflect the fact that it is distinct from CS clinically and because it is caused by a very specific mutation. Crouzonodermoskeletal syndrome is similar to classic CS, but in addition to the craniosynostosis and facial features noted above, patients develop acanthosis nigricans by puberty. Acanthosis nigricans is a skin condition that causes areas of dark discoloration in body folds and creases (often the neck, armpits, and/or groin). Affected skin is thicker than other skin and velvety (see photo at right; 20, 21). There may also be hypertrophy of the skin overlying joints. This condition does not occur in CS. Patients with this condition have a specific mutation in the gene FGFR3, and genetic testing can be helpful in distinguishing the two conditions (19). In addition, people with Crouzonodermoskeletal syndrome have distinctive alterations of the vertebral column, and many develop jaw tumors called cementomas (19).
Apert syndrome (AS). AS is very similar to Crouzon syndrome. People with both conditions have craniosynostosis, wideset eyes, proptosis/protruding eyes, and dental abnormalities (22). Unlike Apert syndrome, however, Crouzon syndrome does not cause severe syndactyly of the the hands and feet. Given that this problem is universal and severe in AS, its presence or absence can help distinguish the two conditions. In addition, most patients with Apert syndrome have intellectual disabilities (22) while a majority of CS patients do not (6).
Pfeiffer syndrome (PS). Pfeiffer syndrome is another craniosynostosis syndrome. It is also very similar to CS. People with PS have craniosynostosis, foot syndactyly, proptosis/protruding eyes, highly arched palates and dental abnormalities. They may also have facial asymmetry and downslanting palpebral fissures. Intellectual disability occurs in PS, but intelligence is normal in the majority of patients (7). People with PS may have cloverleaf skulls (see photos on PS page), while this condition does not generally seem to appear in CS. In addition, people with PS have broad, medially deviated thumbs and toes, while people with CS do not (6). Short fingers and toes (brachydactyly) are also common in PS but not CS (6).
Jackson-Weiss syndrome (JWS). JWS is a very rare member of FGFR-related craniosynostosis syndromes group. It was reported in a large Amish kindred in 1976 (23). As with Crouzon syndrome, people with JWS and AS have craniosynostosis, wideset eyes, proptosis, and foot abnormalities. Like Crouzon syndrome, JWS does not generally cause syndacytly of the hands, but syndactyly of the feet may be a distinguishing feature. Foot abnormalities are not characteristic of Crouzon syndrome, though they are common in JWS. Relatively few studies have reported on JWS; the largest did not find evidence of intellectual disability, although it has been reported in a smaller study (24).
Saethre-Chotzen syndrome (SCS). Although SCS is a syndrome involving craniosynostosis, it is not a member of the FGFR-related craniosynostosis syndromes group, because it is caused by mutations in the gene TWIST1. SCS is an autosomal dominant disorder where, as in Crouzon syndrome, most people with SCS have an affected parent (25). The clinical features of SCS vary, even in affected members in the same family. Severity ranges from mild to severe. Most people who have SCS have craniosynostosis, although the cranial cutures that are fused vary. Strabismus (misaligned eyes) is also common. Syndactyly may be present, but it usually affects only two fingers. In addition, the cosmetic features of SCS are usually mild in comparison with other craniosynostosi syndromess, and most SCS patients have normal intelligence. Severe intellectual disability appears to be rare in SCS (25), as in CS (6). Genetic testing can differentiate SCS and CS, however, because they are caused by different genes.
References
- 1. Cohen MM & MacLean RE, eds. (2000) Craniosynostosis: Diagnosis, evaluation, and management. New York: Oxford University Press.
- 2. Reefhuis J et al. (2003) Fertility treatments and craniosynostosis: California, Georgia, and Iowa, 1993-1997. Pediatrics 111(5 Pt 2):1163-1166. Full text from publisher.
- 3. Boyadjiev SA et al. (2007) Genetic analysis of non-syndromic craniosynostosis. Orthod Craniofac Res 10(3):129-137. Abstract on PubMed.
- 4. Alderman BW et al. (1988) An epidemiologic study of craniosynostosis: risk indicators for the occurrence of craniosynostosis in Colorado. Am J Epidemiol 128(2):431-438. Abstract on PubMed.
- 5. Carmichael SL et al. (2008) Craniosynostosis and maternal smoking. Birth Defects Res A Clin Mol Teratol 82(2):78-85. Abstract on PubMed.
- 6. Robin NH et al. (1998) FGFR-Related Craniosynostosis Syndromes. Updated June 7, 2011. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 7. Wilkie AO (1997) Craniosynostosis: genes and mechanisms. Hum Mol Genet 6(10):1647-1656. Abstract on PubMed.
- 8. Cohen MM Jr et al. (1992) Birth prevalence studies of the Crouzon syndrome: comparison of direct and indirect methods. Clin Genet 41(1):12-15. Abstract on PubMed.
- 9. Tuite GF et al. (1996) The beaten copper cranium: a correlation between intracranial pressure, cranial radiographs, and computed tomographic scans in children with craniosynostosis. Neurosurgery 39 (4):691-699. Abstract on PubMed.
- 10. Derderian C & Seaward J (2012) Syndromic craniosynostosis. Semin Plast Surg 26(2):64-75. Full text on PubMed.
- 11. da Silva DL et al. (2008). Crouzon's syndrome: literature review. Intl Arch Otorhinolaryngol 12(3):436-441. Full text.
- 12. Connolly JP et al. (2004) Progressive postnatal craniosynostosis and increased intracranial pressure. Plast Reconstr Surg 113(5):1313-1323. Abstract on PubMed.
- 13. Padmanabhan V et al. (2011). Crouzon's syndrome: a review of literature and case report. Contemp Clin Dent 2(3):211-214. Full text on PubMed.
- 14. Bowling EL & Burstein FD (2006). Crouzon syndrome. Optometry 77(5):217-222. Abstract on PubMed.
- 15. Lyons JK, ed. (2006) Crouzon syndrome (craniofacial dysostosis) In Smith's Recognizable Patterns of Human Malformation. Elsevier ISBN-13: 978-0-7216-0615-6 pp. 478-479.
- 16. Goriely A et al. (2010). Germline and somatic mosaicism for FGFR2 mutation in the mother of a child with Crouzon syndrome: implications for genetic testing in "paternal age-effect" syndromes. Am J Med Genet Part A 152A(8):2067-2073. Full text on PubMed.
- 17. Blank CE (1960) Apert's syndrome (a type of aerocephalosyndactyly): observations on a British series of thirty-nine cases. Ann Hum Genet, Lond 24:151-164. Abstract on PubMed.
- 18. Erickson JD & Cohen MM Jr (1974) A study of parental age effects on the occurrence of fresh mutations for the Apert syndrome. Ann Hum Genet 38(1):89-96. Abstract on PubMed.
- 19. Cohen MM Jr. (1999) Let's call it 'Crouzonodermoskeletal syndrome' so we won't be prisoners of our own conventional terminology. (Letter) Am J Med Genet 84(1):74. Abstract on PubMed.
- 20. Wilkes D et al. (1996) A recurrent mutation, ala391glu, in the transmembrane region of FGFR3 causes Crouzon syndrome and acanthosis nigricans. J Med Genet 33(9):744-748. Full text on PubMed.
- 21. Higgins SP et al. (2008) Acanthosis Nigricans: A practical approach to evaluation and management. Dermatol Online J 14(9):2. Full text from publisher.
- 22. Renier D et al. (1996) Prognosis for mental function in Apert's syndrome. J Neurosurg 85(1):66-72. Abstract on PubMed. Full text on ResearchGate.
- 23. Jackson CE et al. (1976) Craniosynostosis, midfacial hypoplasia and foot abnormalities: an autosomal dominant phenotype in a large Amish kindred. J Pediatr 88(6):963-968. Abstract on PubMed.
- 24. Cross HE et al. (1969) Craniosynostosis in the Amish. J Pediatr 75(6):1037-1044. Abstract on publisher website.
- 25. Gallagher ER et al. (2003) Saethre-Chotzen Syndrome. Updated June 14 2012. GeneReviews [Internet] Pagon RA et al., editors. Seattle (WA): University of Washington, Seattle; 1993-2021. Full text.
- 26. Mohan RS et al. (2012) Crouzon syndrome: clinico-radiological illustration of a case. J Clin Imaging Sci 2:70. doi: 10.4103/2156-7514.104303. Full text on PubMed.
- 27. National Human Genome Research Institute. Photos of children with midface hypoplasia. Elements of Morphology: Human Malformation Terminology (Online encyclopedia).
- 28. National Human Genome Research Institute. Photo of person with proptosis. Elements of Morphology: Human Malformation Terminology (Online encyclopedia).
- 29. Lin, Y et al. (2012) FGFR2 molecular analysis and related clinical findings in one Chinese family with Crouzon Syndrome. Mol Vis 18:449-454. Full text on PubMed.
- 30. Fenwick AL et al. (2014) Apparently synonymous substitutions in FGFR2 affect splicing and result in mild Crouzon syndrome. BMC Med Genet 15:95. doi: 10.1186/s12881-014-0095-4. Full text on PubMed.
- 31. Kumar GR et al.(2013) Crouzon's syndrome: a case report. Int J Clin Ped Dent 6(1):33-37. Full text on PubMed.